Full Text
PLASTIC-DERIVED DISSOLVED ORGANIC MATTER
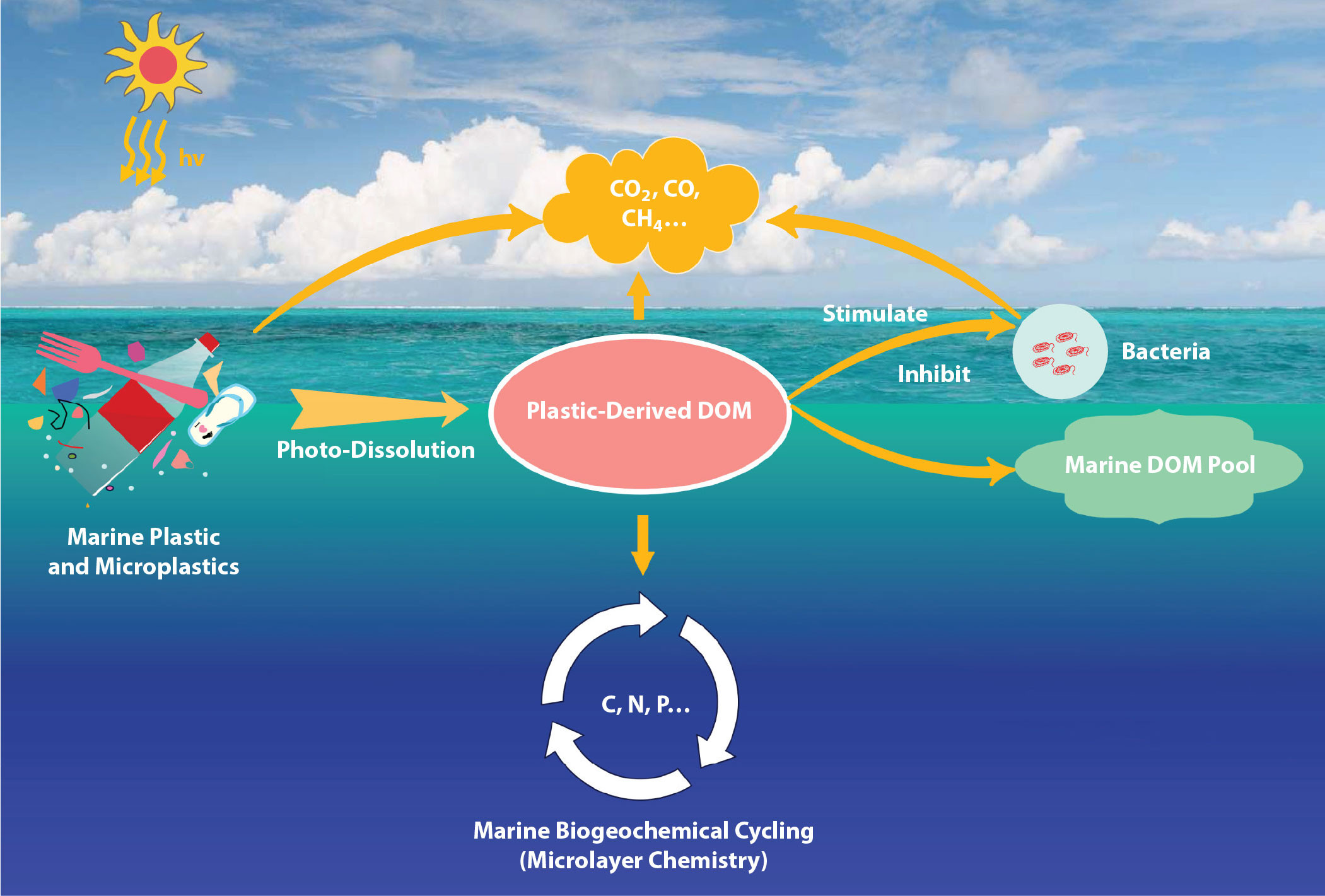
Plastics have accumulated in the environment to become a globally significant pool of organic carbon (Stubbins et al., 2021) and a contaminant of ecological concern (McLeod et al., 2021). Most studies still report plastics in terms of counts (i.e., pieces of plastics) though some report masses of plastics, and even fewer report plastic carbon. In a recent review, we assumed plastics to be 83% carbon by mass based on data for oceanic microplastics (Zhu et al., 2020; Stubbins et al., 2021). This overly simplistic conversion allows plastics to be placed in a carbon cycle context, a context critical to plastic-derived dissolved organic carbon (DOC; Figure 1). However, this conversion should be improved to account for variations in the carbon content of different polymers. To make these improvements, studies should report data for sizes, masses, and types of base polymer of plastics collected. These data would allow comparison among studies and facilitate improved accounting for plastics and their fates in the environment. Reporting masses of specific polymers would make conversion from plastics to carbon more accurate and provide a common chemical unit for comparison among plastic polymer types (i.e., we could use carbon as we do biomass).
|
|
From a geochemical perspective, plastics are ultrahigh molecular weight organic geomaterials that also carry other organics into aquatic ecosystems (e.g., additives and other lower molecular weight organics adsorbed to the plastics). Once plastics enter aquatic ecosystems, they can leach organic substances that include additives such as plasticizers (e.g., phthalates), colorants, and flame retardants (Gewert et al., 2015) as well as adsorbed chemicals such as organic pollutants and natural organic matter. If these molecules are leached from plastics into water, they enter the pool of dissolved organic matter (DOM, defined as organic matter that passes through a filter of 0.2–0.7 µm; Bracchini et al., 2010; Dittmar and Stubbins 2014). In addition to leaching from passenger molecules, DOM can derive directly from plastic polymers that are degraded in natural waters via photo-dissolution by sunlight (Romera-Castillo et al., 2018; Zhu et al., 2020). Photo-dissolution, which results from this scission (photochemical “cutting” of bonds in the polymer) and oxidation reactions, produces a suite of soluble low molecular weight products (Eyheraguibel et al., 2017). Sunlight can completely dissolve microplastics floating at sea in 0.3 to 50 years, depending on polymer type, and the kinetics employed in modeling suggests all floating microplastics at sea would dissolve within decades if inputs ceased (Zhu et al., 2020).
The mass of microplastics floating at or near the sea surface amounts to 93–236 thousand metric tons (Van Sebille et al., 2015) or 0.07–0.20 Tg plastic-C (Stubbins et al., 2021). If all this plastic were to dissolve, it would constitute a tiny fraction of total ocean DOC stocks (662,000 Tg-C; Hansell et al., 2009). However, because plastic-derived DOM is produced in sunlit surface waters, the addition of DOM could impact the ecology and biogeochemical functioning of surface waters. Photoproduced plastic-derived DOM contains transphilic products such as carboxylic acids that may fractionate into the marine microlayer and impact trace gas and aerosol exchange with the atmosphere and with surface ocean ecology and biogeochemistry (Boldrini et al., 2021; Galgani and Loiselle, 2021). In most cases, the DOM produced as plastics photo-dissolve is highly biolabile and utilized by bacteria, thus causing no obvious adverse effects (Romera-Castillo et al., 2018; Zhu et al., 2020). However, dissolved photoproducts from one sample of polyethylene inhibited microbial growth (Zhu et al., 2020), indicating some plastic-derived DOM may be cytotoxic. Whether this is true for other types of plastic-derived DOM or at environmentally relevant concentrations remains unclear.
Although there is broad concern about the impacts of microplastics in natural waters (Macleod et al., 2021), there is limited understanding about the production and impacts of plastic-derived DOM. To aid efforts to gain greater understanding, we present some of the tools used to study DOM, including their applications to plastic-derived DOM.
LABORATORY STUDIES OF PLASTIC-DERIVED DOM PHOTOPRODUCTION
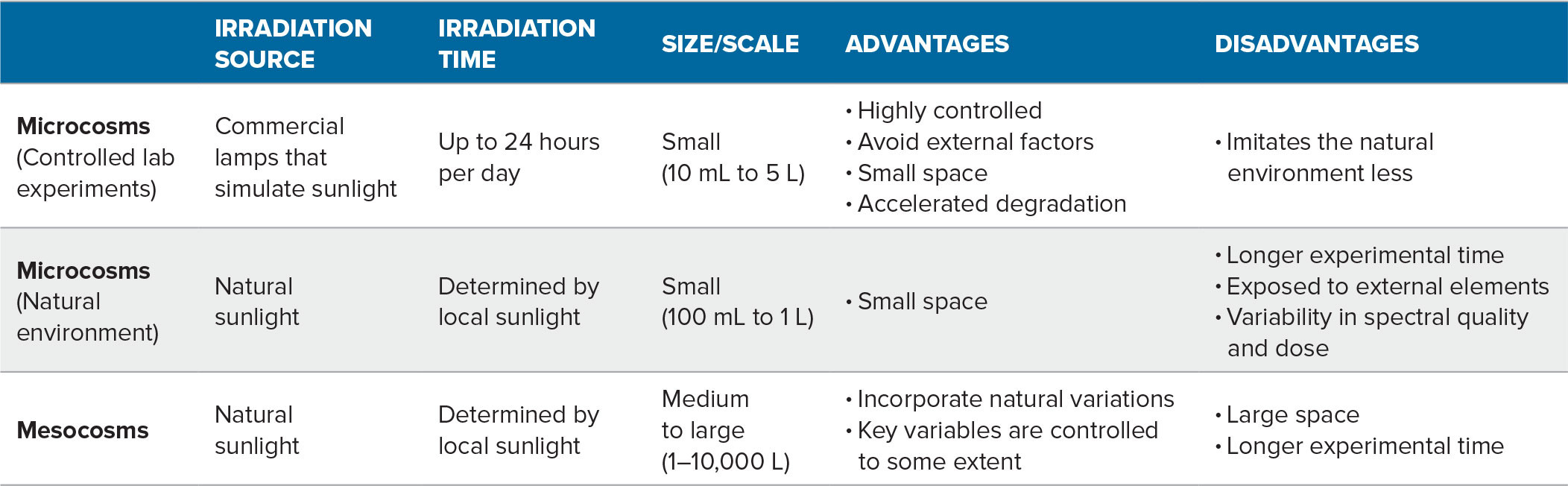
Because direct measurement of environmental photochemical rates in natural waters is challenging, rates are usually determined in more controlled settings and then extrapolated to the field (e.g., Mopper et al., 2015). To reduce the number of variables, but still maintain the functionality of key drivers, photochemical microcosm and mesocosm studies are employed, with irradiation times varying from hours to years. For all types of experiments discussed below, plastics should be thoroughly cleaned, and low DOM background water should be used. In studies seeking to assess abiotic photochemical rates, bacterial abundance should be monitored to ensure samples remain sterile and DOM is not altered or consumed prior to analysis. Studies seeking to understand the coupling of photochemical and biological processes do not need to be sterile.
Microcosms
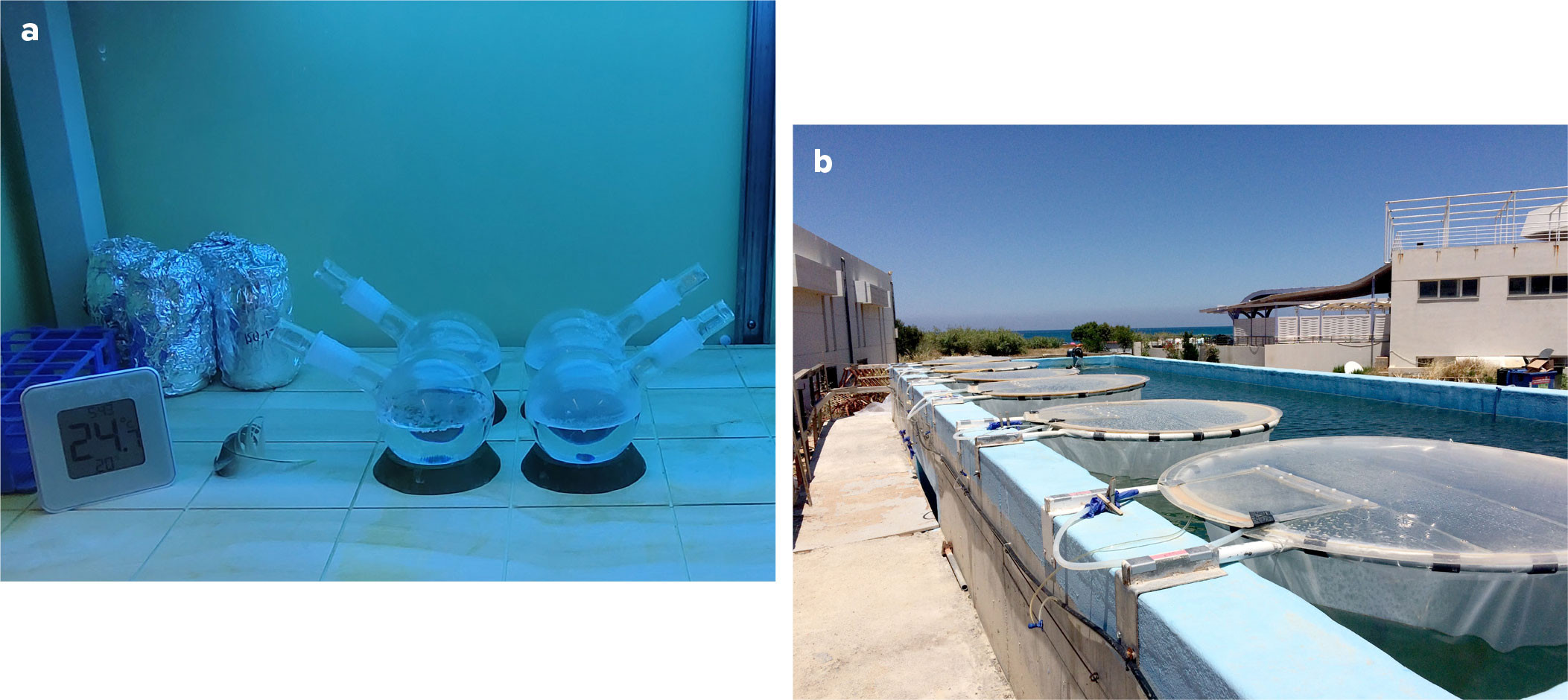
Microcosm experiments explore a limited number of variables under controlled conditions (Table 1). The photo-dissolution of plastics is assessed in quartzware, as quartz allows environmentally relevant wavelengths of ultraviolet (UV) light (>280 nm) to enter. This wavelength range is responsible for most environmental photochemical reactions (Loiselle et al., 2009a; Mopper et al., 2015). One common microcosm is a lab-based solar simulator (Figure 2a), equipped with calibrated commercial lamps (e.g., Q-Panel UVA-340 bulbs) that provide light with a spectral quality and flux approximating the UV radiation in natural sunlight. Integrated irradiance can be quantified via spectroradiometers (e.g., OL 756, Optronic Laboratories), and temperature can be maintained by side mounted fans or water baths.
TABLE 1. Summary of differences between experimental systems for quantification of plastic-derived dissolved organic matter. > High res table |
|
|
Microcosms can also use natural sunlight and quartz flasks in a water bath (e.g., on a rooftop), thus allowing exposure to the natural diurnal dynamics of sunlight. Sunlight irradiance can be measured using UV spectroradiometers (e.g., SKR, 1850; SKR 420, 430; Skye Instruments LTD, UK). The spectral incident irradiance (Eo (λ, t), kW m−2 nm−1) is calculated by considering total solar irradiance on the circular mesocosm surface and the spectral transmission of the quartz (Loiselle et al., 2012). For example, DOM production from the degradation of different plastic bags was assessed by cutting them into 10 cm2 squares, weighing and analyzing the plastic via Fourier transform infrared spectroscopy (FTIR), then floating them in quartz microcosms. Temperatures over a summer averaged 30°C, and light exposures matched those of unshaded local waters.
DOM samples should be collected through time to assess the kinetics of plastic-derived DOM accumulation. Extended periods of irradiation outdoors increase the possibility of complications due to extreme weather events and biofouling of the external quartz surface. Whether in the lab or outside, it is important to define, a priori, whether samples are to remain sterile or exposed to typical microbial conditions, as biolabile DOM will be modified by both photodegradation and microbial degradation and production processes (Galgani et al., 2018).
Mesocosms for Photochemistry
Larger-scale mesocosm (usually 1–10,000 L) experimental systems are set up in natural environments and provide a link between field and microcosm experiments. While still controlled, they are more exposed to environmental influences (Table 1, Figure 2b). Irradiance, temperature, and other factors are monitored using sensors, while regular sampling at different depths allows improved understanding of the processes mediating production, loss, and transformation of plastic-derived DOM in natural conditions. Mesocosms were used to determine the impact of microplastics on microbial DOM production by enhancing the production and accumulation of two important classes of marine gels in the sea surface microlayer (Galgani et al., 2023) and in bulk waters.
For mesocosm experiments, contamination with DOM from external sources should be avoided. In Galgani et al. (2019), mesocosms were filled with 3 m3 of coastal oligotrophic seawater pumped directly from the Sea of Crete. The mesocosms were constructed of transparent polyethylene that was previously tested to ensure they did not release DOM. These mesocosms were housed in a concrete tank (150 m3) with circulating seawater to maintain temperature (20° ± 1°C). After 24 hours, homogeneity between mesocosms was assessed. Transparent polystyrene microbeads (Sigma-Aldrich, nr. 84135; density of 1.05 g cm–3) were added to three mesocosms (430 beads L–1); polystyrene beads were chosen to reduce the possibility of attraction to the polyethylene walls of the mesocosm (Beneš and Paulenová, 1973). A centralized airlift system (Pitta et al., 2016) assured homogeneous distribution of the microplastics. Following Carlson (1982), the surface microlayer was sampled every other day using acid washed and Milli-Q rinsed glass plates, collection bottles, and wipes for phytoplankton sampling in the microlayer. Bulk water was sampled from 0.5 m depth using acid-washed and Milli-Q rinsed tubes. To avoid cross-contamination, different sampling equipment (glass plates, funnels, collection bottles, wipes) was used for the control mesocosms and the microplastic-treated mesocosms. Mesocosms containing microplastics showed increased production of DOM in both bulk waters and surface microlayers and an increase in the production of carbohydrate-like and proteinaceous marine gel compounds in the surface water (Galgani et al., 2019). Implications of this increased layer of organic material at the sea surface concern the transport of microplastics out of the photic zone and gas exchange across the sea surface.
EXPERIMENTAL CONSIDERATIONS FOR PLASTIC-DERIVED DOM INTERACTIONS
Experiments have been conducted using standards, commercial products, and environmentally collected plastic debris. All plastics should be cleaned and dried to avoid contamination. Cleaned plastics can be cut into various sizes or shapes. The cleanness of the plastics can be checked under a microscope. Prior to transferring to mesocosms or microcosms, all plastic samples should be identified for polymer type, weight, dimension, and number.
Plastics are added to water to assess DOM production and leaching. Natural seawater, artificial seawater, freshwater, or ultraclean water (e.g., Milli-Q) can be used. The amount of photochemically active background DOM in natural waters can be lowered by irradiating under germicidal ultraviolet-C light (Zhu et al., 2020). Milli-Q water has a near-zero DOM background, making it easier to quantify DOM production from plastics. However, the mechanisms of DOM release in Milli-Q do not reflect those of natural waters. Natural water contains DOM and other photo-active solutes (e.g., chloride, bromide), as well as microbes that can colonize plastic surfaces as biofilm and transform released DOM. Both light and dark controls are recommended. Light control samples are irradiated in the same way as the experimental samples that do not contain plastics. Two types of dark controls should be included. The first includes plastics, while the second should have no plastics, and both should be kept in the dark (e.g., foil wrapped but otherwise in the same location and condition as the light samples).
The amount of plastic sample used depends on the objective. To best mimic natural processes, the concentration and surface area of the plastic should be comparable to that found in the environment. For example, 240 particles per liter were used in Zhu et al. (2020). However, to obtain enough plastic-derived DOM for further analyses or experiments, or to explore kinetics and mechanisms, higher concentrations of plastics can be used. In microcosms, flasks can be repositioned daily to average out potential spatial variation in the light flux. Throughout the irradiation period, liquid samples can be drawn from the flasks using pre-combusted glass Pasteur pipettes at regular intervals. Duplicates or triplicates are advised. At the end of the experiments, plastic fragments can be dried, weighed, and further analyzed for carbon content using an elemental analyzer. Changes in surface properties can be accessed via optical and electron microscopy, as well as FTIR. All sample handling and experimental procedures should follow trace clean protocols for natural DOC analysis (Stubbins and Dittmar, 2012). All plasticware should be cleaned by triple rinsing with Milli-Q water, soaked overnight in ~pH 2 water, triple rinsed with Milli-Q, and then dried. Glassware and quartzware should be cleaned in the same way and then combusted at 450°C for 6 hours to remove any trace organics. Ideally, the bacterial abundance throughout the experiments should be monitored. Numerous techniques exist for this, including flow cytometry (Zhu et al., 2020).
DOM QUANTIFICATION AND PROPERTIES
Plastic-derived DOM generated during incubation in microcosms or mesocosms can be qualified and quantified in the following ways.
Dissolved Organic Carbon
DOM is generally quantified as dissolved organic carbon (Dittmar and Stubbins, 2014). Samples are filtered and then acidified to pH <2 using hydrochloric acid before analysis using a TOC analyzer (e.g., Shimadzu). For marine work, certified DOC Consensus Reference Materials (CRM, University of Miami; https://hansell-lab.earth.miami.edu/_assets/pdf/lc-table-1-consensus-values-for-doc-and-tdn_2021.pdf) should be used to confirm accuracy and consistency with the international marine DOC community. Routine minimum DOC detection limits are 0.034 mg C L–1, and standard errors are typically 1.7 ± 0.5% of the DOC concentration. The accumulation of plastic-derived DOC can be normalized to the initial plastic weight, initial plastic carbon weight, or initial plastic surface area.
Optical Properties of DOM
The optical properties of DOM are measured via absorption or fluorescence spectrophotometry (Dittmar and Stubbins, 2014). Samples are filtered and stored in the dark, but not acidified or frozen, and are ideally measured within a day of sampling.
Absorbance spectra (typically from 230 nm to 750 nm), including the spectral slope, provide information about the chemistry (e.g., aromaticity, molecular weight), origin, and degradation of DOM in the environment (Helms et al., 2008; Loiselle et al., 2009b). Fluorescent excitation-emission matrix spectra (EEMs) give information about DOM quality, including signatures that may correspond to DOM photo- and biolability (Coble, 1996; Dittmar and Stubbins, 2014; Fellman et al., 2010).
When sealed quartz cuvettes (10 cm path length, Hellma 120-QS, Quartz SUPRASIL, Helma Analytics) are used as microcosms, absorption can be analyzed without invasive sampling by positioning the cuvettes in a spectrophotometer and then returning them to the experimental conditions (Galgani et al., 2018). Plastics used in these circumstances should have densities sufficiently different from water so the plastics do not remain in the light path of the cuvettes. As biofilms may form on the cuvette surface, cells should be cleaned prior to analysis. Monitoring changes in CDOM absorbance in this way is a low cost, low impact method for assessing the impacts of plastics on aquatic DOM.
Ultrahigh-Resolution Mass Spectrometry
Ultrahigh-resolution mass spectrometry resolves the molecular complexity of DOM. Fourier-transform ion cyclotron resonance mass spectrometry (FT-ICRMS) coupled with soft ionization techniques such as electrospray ionization (ESI) is used to simultaneously detect the exact mass of tens of thousands of molecular ions within DOM samples. Molecular formulas are then assigned to the exact masses. Each identified molecular formula can have numerous structural isomers (Dittmar and Stubbins, 2014).
Nuclear Magnetic Resonance Spectroscopy
To obtain information about the molecular structures present in DOM, solid and liquid state nuclear magnetic resonance spectroscopy (NMR) can be applied (for reviews see Mopper et al., 2007; Dittmar and Stubbins, 2014). The NMR approach depends upon what information is desired and the NMR available. For most NMR methods, isolates or concentrates of DOM are required to increase signal strength or separate the DOM from inorganics that degrade signal quality. For seawater samples, low salt isolates are generated via solid phase extraction or reverse osmosis coupled to electrodialysis (Green et al., 2014). Cryoprobes (Kovacs et al., 2005; Bruker 500 MHz spectrometer, equipped with cryoprobe) with water suppression pulse sequences now allow simple, one-dimensional 1H-NMR spectra to be determined directly from seawater samples (McKay, 2011). Plastic-derived DOM is a complex mixture of thousands of molecules that approach natural DOM in complexity. Thus, structural signatures observed by NMR are average structural properties for the suite of molecules present and are not easily resolved to the molecular level (Mopper et al., 2007; Dittmar and Stubbins, 2014).
Support Technologies
The degradation of plastic and the release of plastic-DOM impacts natural waters and the plastics themselves. For waters, continuous measurement of dissolved oxygen can provide important information on microbial activity and oxygen use during plastic-photooxidation. Oxygen content can be measured in gas-tight micro- and mesocosms using self-adhesive sensor spots (e.g., PyroScience, Aachen, Germany) with oxygen probes, or, for high precision, the Winkler method.
Changes in the surface conditions of plastic samples can be determined using scanning electron microscopy (SEM), FTIR, and time-of-flight secondary ion mass spectrometry (ToF-SIMS). SEM provides detailed surface texture information for plastic fragments, while FTIR and ToF-SIMS provide information on the formation of new organic structures (e.g., carbonyl groups) and the incorporation of inorganic elements onto the plastic surface. ToF-SIMS provides spatially resolved chemical composition (Carretti et al., 2022). Simple gravimetric approaches provide an overall indication of material lost during degradation, whereas thermogravimetry can evaluate changes in polymer structure caused by photodegradation (Pashaei et al., 2021).
CONCLUSIONS
Plastics are an emergent organic geomaterial with unknown consequences for the Earth system. As plastics photodegrade in natural waters, they release DOM that may impact marine ecology and biogeochemistry, particularly in the surface microlayer. In this brief review, we introduce some experimental and analytical methods for studying this phenomenon. Some potential impacts of plastic-derived DOM on marine ecosystems are also noted. However, much is unknown about the photochemical processes that dissolve plastics and the chemical products formed. Fortunately, oceanographers have studied DOM for a century and have a mature toolbox of methods for monitoring and modeling these changes. Those wishing to study plastic-derived DOM should become familiar with the broader DOM literature (e.g., Hansell and Carlson, 2014) as well as the environmental photodegradation literature. We provide links to some gateway reviews into the DOM literature that may assist readers who wish to expand the field.