Introduction
Most benthic marine species—particularly coastal species—have a life history that includes a planktonic larval stage and a comparatively sedentary, benthic adult stage. Consequently, these species typically exist in metapopulations, with individual subpopulations of benthic adults occupying habitat patches that are connected only by the movements of larvae, not adults (Cowen and Sponaugle, 2009). The degree of connectivity among habitat patches determines how populations replenish themselves, whether they are demographically “open” or “closed,” and whether they will persist in the face of harvesting or climate change. Thus, efforts to understand the dynamics of marine metapopulations have centered on investigating how ocean currents and larval traits interact to determine transport pathways and the resulting patterns of connectivity among subpopulations.
Twenty years ago, there was a paradigm shift in our understanding of marine population connectivity. While it had been assumed that larvae are essentially passive particles subject to wide dispersal by ocean currents, a series of groundbreaking studies revealed that a surprising proportion of larvae did not disperse, but settled on or near the same reef where they were spawned (reviewed by Swearer et al., 2002). These discoveries were followed by further investigation of the aspects of larval swimming behavior (e.g., Leis, 2007) and nearshore oceanography (e.g., Nickols et al., 2012) that made it possible for larvae to stay closer to home. There have also been theoretical explorations of the implications of greater local retention of larvae for population dynamics and the spatial management of fisheries and marine protected areas (e.g., White et al., 2011). Much of this work was reviewed by several authors approximately 10 years ago (Pineda et al., 2007; Cowen and Sponaugle, 2009); this review will focus on developments since then, and on the current state of the field of connectivity research.
Since 1999, considerable effort has been devoted to unraveling the mysteries of larval connectivity, using tags and genetic methods to actually track larvae through their development and dispersal in the plankton and developing better numerical methods to simulate the ocean currents that transport them (see White et al., 2019, in this issue). We now find ourselves able to return to one of the ultimate goals of connectivity studies: to close the demographic loop and make predictions about population ecology and evolution. Connectivity in the larval stage is only one component of pursuing that overall goal, which also requires an understanding of processes in the benthic environment that affect the number of larvae produced in a given location as well as the survival and growth of juveniles after settlement from the planktonic environment. Elucidating the interplay of all of these elements of connectivity across large spatial scales in the California Current Large Marine Ecosystem (CCLME) has been a central effort of the Partnership for Interdisciplinary Studies of Coastal Oceans (PISCO) since its inception (Menge et al., 2019, in this issue), and this review pays particular attention to work by PISCO investigators.
Here (and throughout this paper), we follow the conceptual framework established by Pineda et al. (2007; Figure 1): larval transport refers to the translocation of larvae from one point to another in the three-dimensional ocean; larval dispersal refers to the movement of a larva from where it is spawned (the “origin” population; we avoid using “source,” as it has other connotations) to where it settles to the benthos (the “destination” population), regardless of the path taken; and larval connectivity describes the process of dispersing and then surviving to enter the adult reproductive population at the destination location (often the process of entering the adult population is termed “recruitment”). What we call “connectivity” has sometimes been termed “realized connectivity” to emphasize the importance of post-settlement processes (e.g., Hamilton et al., 2008); we treat these two terms as synonyms.
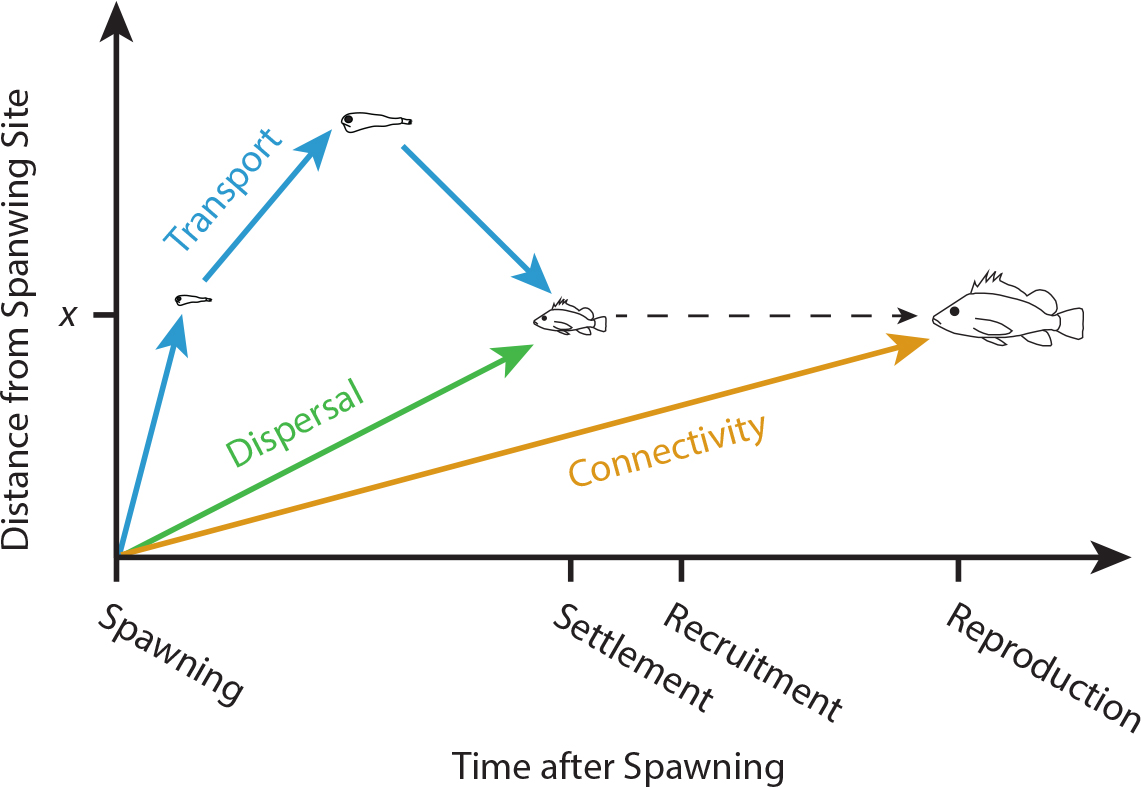
Figure 1. The relationship between the three components of demographic connectivity, from some focal patch to a location distance x away: transport (movement over space, regardless of final destination), dispersal (movement from the focal patch to the patch x units distant), and connectivity (dispersal followed by survival to reproduction). After Pineda et al. (2007). > Full res figure
|
Factors Affecting Larval Abundance and Transport
Transport
Physical processes in the nearshore environment are complex and can either enhance or restrict larval transport (Woodson et al., 2012). Here, we focus on transport processes that disperse larvae away from natal habitats, but many of these same processes can also keep larvae in the nearshore. Much remains to be learned about larval and juvenile retention near those habitats
(Nickols et al., 2015).
The dominant forces affecting transport vary with distance from shore. For larvae traveling close to shore, transport processes include those of the chaotic surf zone along with surface gravity waves and tides (Shanks et al., 2010). Offshore in coastal waters of the inner shelf, transport processes include locally wind-driven currents, internal waves, propagating bores, and movements of steep, horizontal density gradients called fronts. These fronts tend to accumulate eggs, larvae, and juveniles (Woodson et al., 2012). The inner shelf is characterized by overlapping surface and bottom boundary layers where turbulent mixing processes are driven by wind and bottom stresses, respectively (Lentz and Fewings 2012; Figure 2a). These overlapping layers rapidly mix weakly or non-swimming organisms throughout the water column. The rapid vertical transport of momentum through the layers causes currents driven by the wind to tend to move in the wind direction (Austin and Lentz, 2002).
Farther offshore, in the deeper waters of the mid and outer shelf, much larger-scale flows dominate transport: upwelling currents, coastal eddies, and long, slowly propagating coastally trapped waves (Huyer, 1983; Kim et al., 2013; Figure 2). In this region, Earth’s rotation causes ocean currents to flow at right angles to the wind direction. Alongshore flows such as river outflows and buoyant currents associated with wind relaxations can also transport larvae and juveniles across the mid and outer shelf (e.g., Washburn et al., 2011). Beyond the continental shelf, larvae and juveniles enter the deep ocean and are subject to a cascade of ocean currents acting over an extensive range of spatial scales whose sizes range from limbs of the subtropical gyres down to small, open-ocean eddies and submesoscale frontal systems (Siegel et al., 2008). However, larvae that enter those offshore currents are unlikely to return to the coast to settle (Nickols et al., 2015). This latter point is an ongoing difficulty in studying larvae—when sampling larvae over the shelf, it is impossible to know which of them are destined never to return to the coast to settle, due either to bad luck or disadvantageous phenotypes.
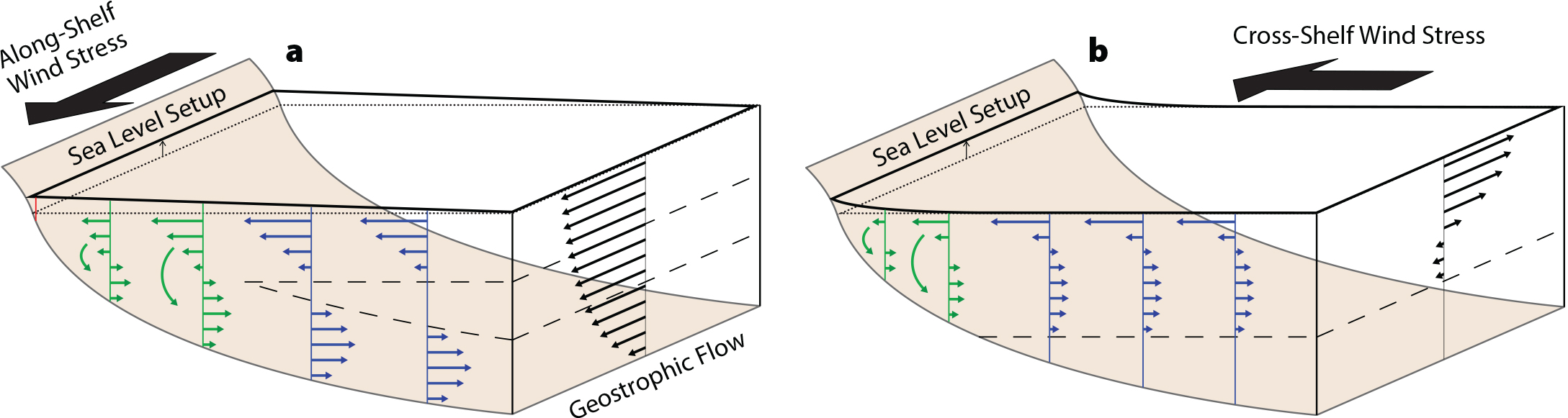
Figure 2. The response of ocean circulation over the continental shelf to prevailing patterns of wind stress. (a) Along-shelf wind stress produces Ekman transport, leading to either upwelling or downwelling, depending on the wind direction, and thus cross-shelf transport (in opposite directions) in the surface and bottom boundary layers (downwelling is depicted here). (b) Cross-shelf wind stress produces along-shelf Ekman transport in the surface boundary layer over the mid to outer shelf. Across-shelf transport is shown in green for the inner shelf and blue for the mid shelf, and the along-shelf current is shown in black. Redrawn from Lentz and Fewings (2012). > Full res figure
|
All of the processes outlined above interact with individual larval behavior to determine where larvae are transported during their pelagic stages (Drake et al., 2013). Of the two ends of the connectivity pathway, the arrival and settlement end of the pathway is easier to study than the outgoing, dispersal end of the pathway. Rapid turbulent dispersion of tiny eggs and larvae following spawning in natal habits complicates both the observation of outbound transport of organisms and the acquisition of time series required to link dispersal of organisms to transport mechanisms. In contrast, arrival and settlement of larvae and juveniles can be quantified as time series by using a host of well-established methods, such as standardized larval collectors of various types (see White et al., 2019, in this issue). This allows, at least in principle, the linking of larval delivery to specific transport mechanisms. Indeed, this approach has revealed how several mid shelf and inner shelf flow phenomena influence settlement of larvae and juveniles in nearshore ecosystems (e.g., Woodson et al., 2012; see section below, Can We Predict Cohort Strength?). Additionally, technical advances in observing larval behaviors have led to improved ability to represent larval behaviors in simulations (Staaterman and Paris, 2013).
The long, central portions of dispersal pathways during transport from the mid shelf into the offshore pelagic environment and then back to the mid shelf are difficult to observe and interpret. Improvements in ocean circulation models, such as the Regional Ocean Modeling System (ROMS), have produced new insights into mesoscale and submesoscale processes that in turn have allowed more realistic simulations of larval transport (e.g., Siegel et al., 2008; Sponaugle et al., 2012; Drake et al., 2013). The ability of circulation models to link offshore transport through the inner shelf to subtidal and intertidal habitats remains an active research area, because great uncertainties remain about the details of larval behaviors and models’ ability to resolve nearshore dynamics. The development of nearshore models that integrate with ROMS, such as COAWST (Coupled Ocean-Atmosphere-Wave-Sediment Transport), are promising tools for simulating larval dispersal from beginning to end (Warner et al., 2010), but uncertainties associated with modeling approaches must be appreciated if their results are to be used in management settings (e.g., Carr et al., 2019, in this issue).
Much remains to be learned about the chain of transport processes that lead from spawning through larval dispersal to eventual settlement into often distant nearshore ecosystems. Many of PISCO’s contributions center on understanding the complex final phases of the connectivity pathways, especially those on the inner shelf (e.g., Caselle et al., 2010b; Woodson et al., 2012). However, these pathways also determine the outgoing or initial transport period, during which traversing the inner shelf may take days or weeks, effectively trapping larvae close to shore and limiting dispersal distances (Nickols et al., 2015). For coastal species, it is likely that processes occurring on the inner shelf are larger contributors to demographic connectivity, and that processes on the mid and outer shelf are more closely linked to less frequent, longer-distance pathways important to evolutionary connectivity.
Abundance and Cohort Strength
Since the early twentieth century, marine ecologists have considered the spatial and temporal variation in larval cohort size (i.e., the number of larvae surviving and recruiting to adult populations each year) to be a primary driver of adult population dynamics (Browman, 2014). Historically, this body of work had two conceptual foci: trophic interactions and bioenergetics (including food availability, larval growth, and predation) and abiotic processes affecting larval transport (see previous section). It has since become clear that the latter strongly affects the former.
An early—and lasting—concept in this field is the “match/mismatch hypothesis” proposed by Cushing (1990), which posited that temporal coincidence of larval production (i.e., spawning) and prey availability was a key determinant of larval survival through the “critical period” after a larva’s energy reserves were exhausted (later extended to include overlap with larval predators as well; Durant et al., 2013). While the overall applicability of the match/mismatch hypothesis continues to be debated, several studies have generated results in its support, including for nearshore rockfishes in the California Current (Wheeler et al., 2017).
A broader view of the match/mismatch concept (going beyond the original focus on the timing of productivity) is that there may be optimal temporal windows in which conditions favor not only prey productivity but also retention in nearshore waters and delivery to settlement habitats (Parrish et al., 1981). In the upwelling-dominated CCLME, this thinking translates into the hypothesis that the timing of larval production of coastal species is linked to the timing of the “spring transition” when winter downwelling-favorable winds change to spring upwelling-favorable winds. The idea is that by timing spawning near this transition, organisms maximize larval retention in nearshore waters and the probability of the larvae returning to adult benthic habitats (Shanks and Eckert, 2005). With growing recognition of the importance of nearshore retention processes to successful recruitment, current work focuses on the interplay among life history traits (e.g., spawning phenology), oceanographic processes (e.g., patterns of upwelling and relaxation), and larval behavior as the drivers of cohort strength (e.g., Shanks and Roegner, 2007; Morgan, 2014).
Other, more explicit hypotheses of larval performance and growth in the plankton motivate current models of recruitment strength. These include the “bigger-is-better,” “growth-mortality,” and “stage-duration” hypotheses (reviewed by Hare and Cowen, 1997). All three emphasize that faster growth rates allow larvae to reduce vulnerability to predation in the plankton, increase foraging success, and return to nearshore adult habitats sooner. These hypotheses integrate both biotic mechanisms such as bioenergetics and larval behavior with physical processes such as fronts and coastal upwelling to predict cohort success. Testing hypotheses that integrate processes across the larval duration is difficult, but it is increasingly being done using coupled biophysical models (e.g., Fouzai et al., 2015).
Can We Predict Cohort Strength?
Long-term monitoring of both physical oceanography and larval settlement in the CCLME has provided several opportunities to test whether the abiotic and biotic factors described in the previous two sections allow us to predict the size of recruiting cohorts of larvae. For example, Shanks and Roegner (2007) found that >90% of interannual variation in the number of Dungeness crab (Metacarcinus magister) larvae collected in Coos Bay, Oregon, was explained by the timing of the spring transition in upwelling winds (i.e., the match/mismatch hypothesis, see Abundance and Cohort Strength section above). That larval collection index, in turn, predicts 90% of the variability in crab catch four years later (when that cohort enters the fishery), and decadal-scale changes in the timing of the spring transition can explain historical trends in the fishery.
In the Oregon intertidal habitat, basin-scale and mesoscale ocean climate indices (e.g., the North Pacific Gyre Oscillation index and Bakun upwelling index)—which describe large-scale trends in both transport processes and productivity over the shelf—explained approximately 40% of spatiotemporal variation in barnacle and mussel larval recruitment. The remaining 60% of variability is apparently due to local-scale transport and species interactions (Menge et al., 2011).
Finally, Caselle et al. (2010a) found that the sizes of larval rockfish (Sebastes spp.) cohorts settling to standardized collectors (see title page photo) in the Santa Barbara Channel Islands (California) were correlated with region-scale transport processes (e.g., Ekman transport) at time lags corresponding to early larval life, but with local-site-scale transport processes (e.g., local wind stress) at short time lags. Separately, Caselle et al. (2010b) found that interannual variability in rockfish settlement to the Channel Islands and in central California was correlated with indices of upwelling and alongshore transport, but with different relationships in each location and across species. Interestingly, those relationships were highly explanatory (R2 ≥0.64) during the mid-2000s (Figure 3a), a stable oceanographic period in the CCLME (e.g., lacking extreme El Niño or La Niña events). These predictive relationships have broken down in recent years (Figure 3b), perhaps in part due to the onset of more extreme and heretofore unusual conditions in the CCLME (Leising et al., 2015). This should be a cautionary tale—past performance does not ensure future success when it comes to predicting larval processes in a changing climate.
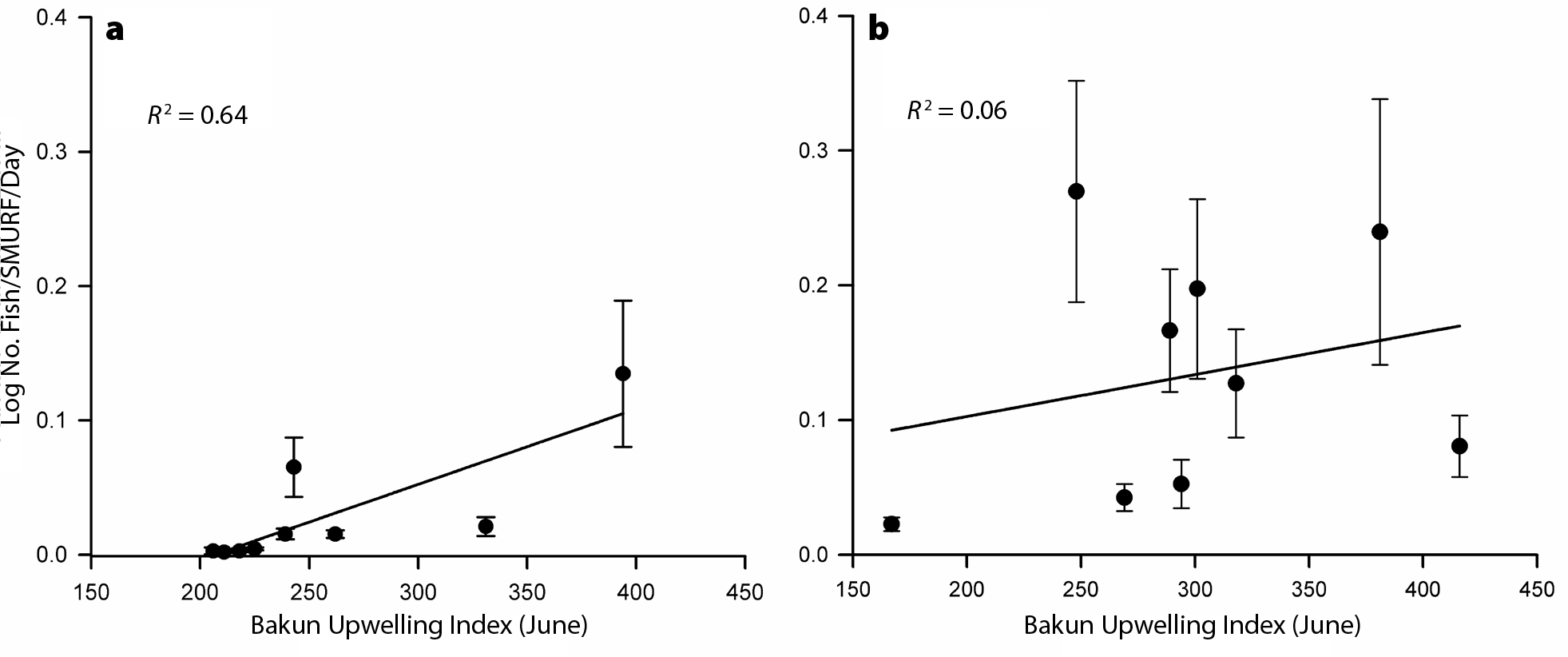
Figure 3. Changing ability to predict larval settlement using oceanographic information. The figure shows the settlement of the “KGBC” complex of rockfishes (kelp, gopher, black-and-yellow, copper, Sebastes spp.) to standardized monitoring units (SMURFs; see White et al., 2019, in this issue) at sites in the eastern Channel Islands, California, as a function of the Bakun index of coastal upwelling in June (measured at the SoCal station, 119ºW, 33ºN). (a) Data from 2000 to 2008 (from Caselle et al., 2010b) show a strong relationship. (b) Data from 2009 to 2017; the relationship disappeared. See Caselle et al. (2010b) for additional details. > Full res figure
|
Closing the Loop for Population Dynamics
As technical advances have made it possible to determine the origin of settling larvae (see White et al., 2019, in this issue), it has also become clear that many species, particularly some coral reef fishes, settle back to their population of origin (Swearer et al., 2002). Estimates of “self-recruitment” (the proportion of settling larvae that were produced locally) have been as high as 96% (reviewed by Burgess et al., 2014). However, it is important to keep in mind what these estimates tell us. Ultimately, high self-recruitment could reflect either isolation (few other nearby populations producing larvae) or a tendency for larvae to remain close to home. The self-recruitment statistic does not, however, reveal the proportion of larvae produced in a population that returns to settle in that population, a statistic termed “local retention.” Notice that these two values have the same numerator (number of settlers produced in population X) but different denominators (number settling in population X vs. total number of larvae produced in X). Only the latter provides direct insight regarding population replacement and persistence (Burgess et al., 2014; see Box 1).
Box 1. Population Dynamics and Connectivity: Lessons from Theory
Understanding the consequences of larval dispersal and connectivity for population dynamics centers on the fundamental concept of replacement. For a population to persist, each individual must—on average—replace itself during its lifetime with at least one successful offspring. When discussing replacement, it is helpful to refer to the lifetime egg production (LEP) of an individual: the average number of offspring (eggs) it will produce over its lifetime, given the probability of survival to each successive age and the increases (or decreases) in fecundity with age.
For a marine population with high local retention of larvae, it is straightforward to see how the replacement concept works: the replacement criterion will be satisfied if LEP multiplied by the probability of local retention and the probability of survival during the larval stage until recruitment is greater than or equal to 1. This is termed “self-persistence” (Burgess et al., 2014). However, in many (most?) cases, local retention for a particular habitat patch or subpopulation will be far too low for self-persistence. Such a subpopulation would only persist if (a) it is completely subsidized by larval supply from another self-persistent subpopulation, or (b) it is “network persistent.” In network persistence, replacement occurs over multiple generations. For example, larvae from patch A disperse to patch B, mature and reproduce, and some of those larvae (the “grandchildren” of the first generation) return to patch A. For a set of subpopulations to be network persistent, the shortfall in single-generation self-replacement is made up for by replacement through one or more of those multi-generation loops (Burgess et al., 2014).
In practice, one could use estimates of connectivity among patches to determine whether any are self-persistent, and whether there are subnetworks of patches that are network persistent (Garavelli et al., 2018). There are clear implications of this for marine spatial planning, for example, determining if a marine protected area will support a self-persistent population or if a protected area network is network persistent (Burgess et al., 2014).
The other major effect of connectivity on population dynamics has to do with synchrony. Greater connectivity will cause subpopulations to have correlated fluctuations in response to environmental variability. This could be unfortunate from a conservation standpoint, because the entire metapopulation would be more stable if subpopulations fluctuated independently (Hilborn et al., 2003). On the other hand, greater connectivity will tend to allow faster recolonization of habitat patches that suffer disturbance, and so could afford greater population stability in that sense (Kallimanis et al., 2005)
As it has become possible to determine dispersal pathways in marine populations (e.g., Baetscher et al., 2019), there has been interest in determining whether patches are demographic “sources” or “sinks.” This idea has its roots in terrestrial ecology, where patches may have a net positive (source) or net negative (sink) growth rate. The idea is more difficult to apply in marine systems in which offspring produced in one patch primarily disperse to other patches (Figeuira and Crowder, 2006): does that make such a patch a source (high net reproduction) or a sink (would go extinct in isolation)? A better understanding of how population persistence operates in such connected networks (Box 1) suggests that the source/sink distinction is not productive. In a marine spatial planning context (e.g., Carr et al., 2019, in this issue), it is sensible to estimate the relative contribution of a patch to persistence (or perhaps fishery yield), but such calculations require modeling the dynamics of the entire metapopulation rather than focusing on a single patch. Additionally, understanding the role of a patch in the metapopulation cannot rely on dispersal probabilities alone (such as those derived from a numerical circulation model); rather, patterns of larval production and post-settlement survival must be incorporated as well (Garavelli et al., 2018; Figure 4).
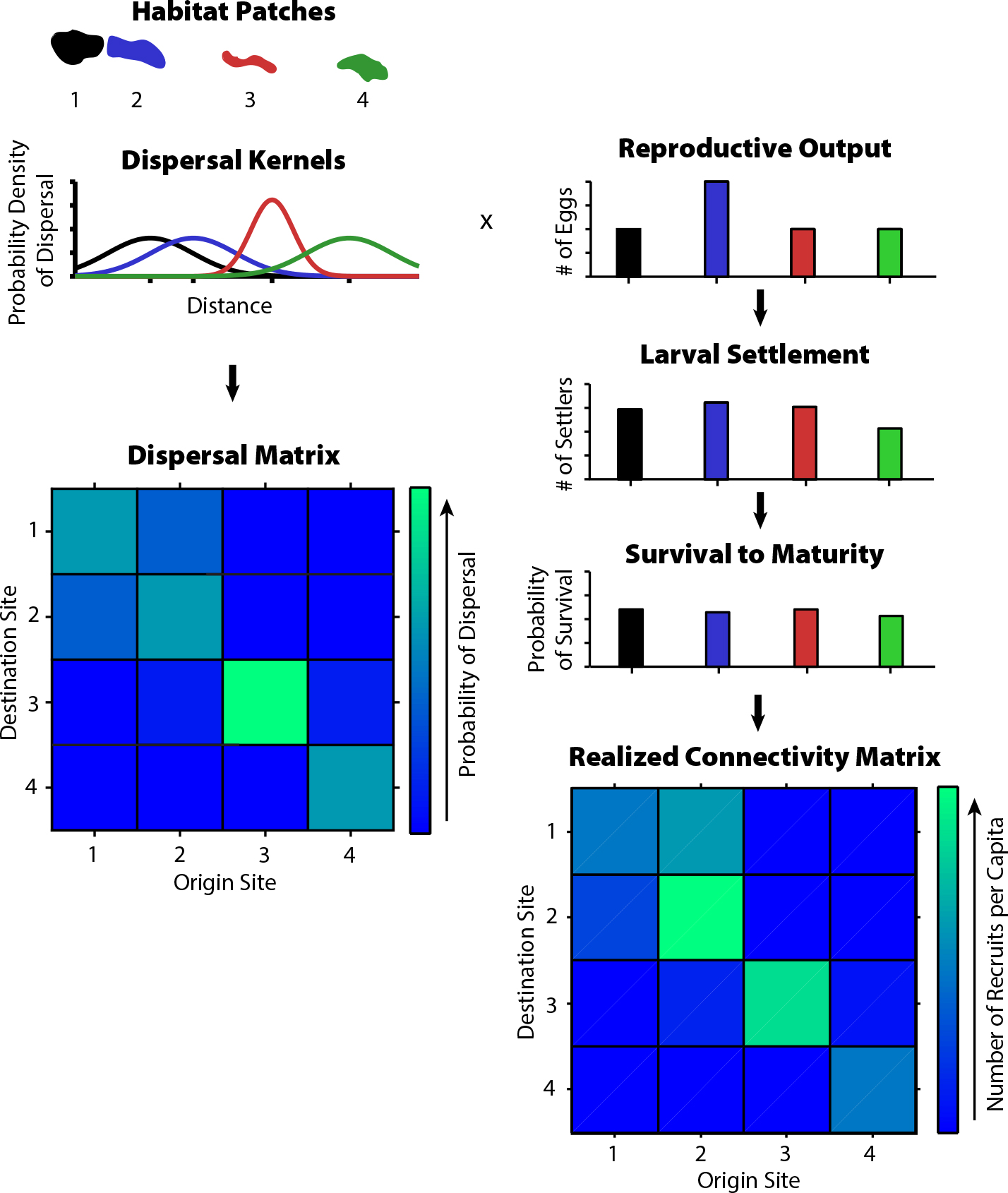
Figure 4. The difference between dispersal matrices (and kernels) and connectivity matrices. The left column shows four islands and the hypothetical dispersal kernels (giving the probability density function of larval dispersal from each island to any other location) for each; the resulting dispersal matrix gives the probability of dispersal from each island to each other island. Accounting for spatial differences in larval production and settler survival (right column) yields the connectivity (number of successful recruits per spawning adult in the origin patch). The “x” indicates that dispersal probabilities are multiplied by reproductive output to obtain settlement, which is then multiplied by survival to obtain realized connectivity. Notice that larvae from patch 2 have only a moderate probability of returning to 2 (left panel, matrix diagonal) but that patch has the highest self-connectivity (right panel) and is thus most likely to be self-persistent. Based on Burgess et al. (2014). > Full res figure
|
There is also a growing appreciation of the importance of the stochastic nature of transport, and thus connectivity. Because ocean circulation is fundamentally chaotic and turbulent, and the movement of individual water parcels is autocorrelated, larval transport will be fundamentally random and unpredictable from year to year (Siegel et al., 2008). The effect on populations is that replacement (Box 1) and genetic connections will occur via sporadic pulses rather than steady streams. The implications for the persistence of benthic populations and communities have been described in terms of the “storage effect,” in which the effects of recruitment pulses and droughts are smoothed out over the reproductive lifetime of an organism (see Warner and Chesson, 1985), but the consequences of this variability also need to be accounted for in the adaptive spatial management of coastal populations (Carr et al., 2019, in this issue).
Importance of Variation in Larval Production
Historically, the relationship between larval production and recruitment has been investigated at broad spatial scales (e.g., entire fishery stocks), with little attention to the role of spatial variation in production for explaining spatial variation in recruitment. This limited attention reflected the perceived decoupling of local production from local recruitment by long distance dispersal, the high rates of larval mortality (99%), and the poor stock-recruitment relationships measured for fisheries stocks. Even at the broad stock-wide spatial scales at which fisheries ecologists study recruitment, attention has focused much more on factors that explain variable survival of larvae (see above section on Abundance and Cohort Strength). However, this neglect may be misdirected.
Fundamentally, larval production is a function of the population size, the size and age distribution of those individuals, key life history traits (e.g., size at maturity, multiple brooding) and female condition (energy available for gonadal production). The importance of size and age structure is underscored by recent evidence for the disproportionate fecundity of large female fish (e.g., Barneche et al., 2018). These consequences of size and age-dependent fecundity likely contribute to the difficulty in discerning a clear relationship between spawning biomass and recruitment at stock-wide scales (He et al., 2015). More accurate means of estimating actual larval production and how maternal provisioning to eggs and larvae influence recruitment are likely to increase our appreciation for explanatory power of larval production for connectivity.
Spatial variation in larval production is receiving greater attention in the context of conservation and fisheries management. Spatial patterns of larval production are a key element of spatially explicit larval connectivity models for the evaluation of both the design and the performance of networks of marine protected areas (MPAs; Carr et al., 2019, in this issue). In addition, changes in environmental conditions associated with global climate change (e.g., water temperature, oxygen content) will influence patterns of larval production, including the redistribution of populations, adult growth, and the relative allocation of energy to gonadal and somatic production (Gerber et al., 2014).
Importance of Post-Settlement Survival
The patterns of abundance established at the time of larval settlement can be modified greatly immediately after settlement, either due to competition for space or predator refuges, or to spatiotemporal variation in physical stress or the abundance of natural enemies (White et al., 2010; Menge et al., 2011). What is particularly challenging from a connectivity standpoint is the growing evidence that a settling larva’s post-settlement mortality risk depends on its condition at settlement, because lower-condition fish may undertake riskier behaviors (e.g., Dingeldein and White, 2016). Condition at settlement, in turn, is affected by the oceanographic conditions a larva encounters (e.g., Shima and Swearer, 2010). Because larvae from different origins will likely encounter different conditions during transport to a given settlement site, their differential condition—and thus survival—at settlement could alter the relative contribution of each origin site to the new cohort (Hamilton et al., 2008). This realization has informed our view that the concept of connectivity (Figure 1) must include post-settlement factors such as origin-dependent selective mortality, rather than simple transport probabilities alone.
Consequences of Benthic Effects on Population Connectivity
Linking spatial patterns of production, transport, and survival—closing the loop—is essential to determining how metapopulations persist (Burgess et al., 2014), the relative value of each patch to metapopulation persistence, and patterns of resilience (Watson et al., 2011). In a multi-species context (e.g., predator-prey interactions), there are opportunities to understand metacommunities and meta-ecosystems as well (White and Samhouri, 2011). This type of information is key to informed marine spatial planning and adaptive management, particularly of marine protected areas (e.g., White et al., 2011). Recently, Johnson et al. (2018) used information on production, transport (based on parentage), and survival to actually make the necessary calculations (see Box 1) to estimate population persistence over different spatial scales for a coral reef fish. Similarly, Garavelli et al. (2018) combined circulation model predictions with a population model to predict that some Caribbean nations could support self-persistent populations of spiny lobster, despite the basin-wide scale of dispersal in that species. Given a growing appreciation for the importance of all stages of the connectivity process—and new technological advances—we anticipate a growing number of studies will be able to apply connectivity theory (Box 1) to connectivity data.
Eco-Evolutionary Consequences of Connectivity
So far in this article we have focused on the demographic implications of connectivity for population dynamics. However, connectivity is also important to the potential for evolutionary divergence of distant populations, due either to genetic drift or selection. The large number of loci analyzed in modern genome-wide analyses allow much finer detection of subtle patterns of gene flow that reveal barriers to connectivity among distant populations. They also provide a much higher probability of detecting loci that are under active selection in different environments. For example, abalone (Haliotis spp.) along the North American west coast show very few differences in gene frequencies despite their low dispersal potential. This has been ascribed to their large population sizes, which means that just a little migration along the coast can be a stronger evolutionary force than local genetic drift (e.g., Gruenthal et al., 2007). Yet, when abalone populations were compared across locations that differed in exposure to stressors (low pH and hypoxia) and before versus after a mass die-off, many loci likely to be under strong selection had different gene frequencies (De Wit et al., 2014). This type of pattern renders it problematic to infer connectivity patterns from genetic patterns because a genetic difference between populations may not reflect a lack of dispersal but instead different habitats selecting for different traits (see section above on The Importance of Post-Settlement Survival).
Conclusion and Future Directions
This paper emphasizes the progress made in understanding larval transport processes, and the importance of linking that information with benthic processes to understand the whole connectivity cycle. What comes next? Figure 5 illustrates some of the key knowledge gaps that remain. Increasingly, we realize the importance of variability, both over space in larval production and retention and post-settlement survival, and also over time in transport pathways and the timing of key oceanographic events. Moreover, that variability will likely increase as the climate changes in future years. This calls for further attempts to develop a mechanistic understanding of the factors leading to predictability (or not?) in cohort sizes. In the CCLME, many species spawn near the onset of upwelling season, a time of pronounced alongshore current reversals over the inner and mid shelf. Better understanding of how the timing, frequency, and magnitude of upwelling will change over time (and in response to climate variability such as the El Niño-Southern Oscillation) will remain an important goal. Additionally, as we develop better models of larval transport, we will likely find that the pathway taken by dispersing larvae is also important, given high variability in the distribution of prey, predators, and unfavorable ocean conditions (e.g., the hypoxic and low pH conditions that are pronounced in the CCLME, especially during upwelling).
In an applied context, linking models and data that describe both benthic and pelagic processes is key to understanding how marine spatial planning and MPAs will affect population and community dynamics. The past decade has seen major advances on this front for MPA planning (Carr et al., 2019, in this issue); the next will likely see the implementation of connectivity models for MPA evaluation and adaptive management. Additionally, there is a need to understand how both connectivity and population dynamics will respond to climate change (and how management should respond). While some simple predictions have been made, the potential eco-evolutionary response—in terms of adaptive shifts in traits that affect connectivity—is still largely unknown. As we address these challenges in the CCLME and globally, the long-term, large-scale data sets of the type collected by PISCO for the past 20 years will continue to prove invaluable for detecting trends and testing hypotheses.
Acknowledgments
This work was supported by the David and Lucile Packard Foundation. The authors thank Sabrina Beyers, Mark Morales, Kerry Nickols, Malin Pinsky, Louis Botsford, and three anonymous reviewers for thoughtful discussions and comments that influenced this article.






