Full Text
Marine plankton are an important and diverse group of organisms that make up the lower trophic levels of the marine food web. They play several critical roles in the ocean that have direct or indirect societal benefits, including supporting food security, oxygen production, and carbon sequestration via the biological carbon pump. Plymouth Marine Laboratory (PML) has been making weekly measurements of zooplankton and phytoplankton at Western Channel Observatory (WCO) Station L4 (50°15'N, 4°13'W) since 1988 and 1992, respectively, using traditional ship-based sampling and light microscopy techniques. Thus, Station L4 has become one of the longest-running, continuous plankton time series in the world and a key marine biodiversity reference site for studies into both short- and long-term environmental changes.
Short generation timescales and the potential for rapid changes in community composition make plankton good indicators of environmental change and of the health of the marine environment (McQuatters-Gollop et al., 2024). In the United Kingdom, a Changes in Phytoplankton and Zooplankton Communities indicator has been adopted within the UK Marine Monitoring and Assessment Strategy, which holds to account the UK Marine Strategy for creating a marine protected area (MPA) network and maintaining “clean, healthy, safe, productive and biologically diverse oceans and seas.” The indicator is based on the abundances of distinct planktonic life-forms and their relationships to environmental pressures (Bedford et al., 2020). The same indicator was also used at the regional level in the most recent Convention for the Protection of the Marine Environment of the North-East Atlantic (OSPAR) 2023 Quality Status Report (OSPAR, 2023). Plankton data from the WCO feed directly into both UK and OSPAR reporting. The WCO record is also long enough to support climate change impact assessments. A recent report on long-term changes in plankton communities across the North Atlantic, which included data from the WCO, indicated wide-spread declines in Northeast Atlantic plankton, but more stable levels in the rapidly warming North Sea (Holland et al., 2023).
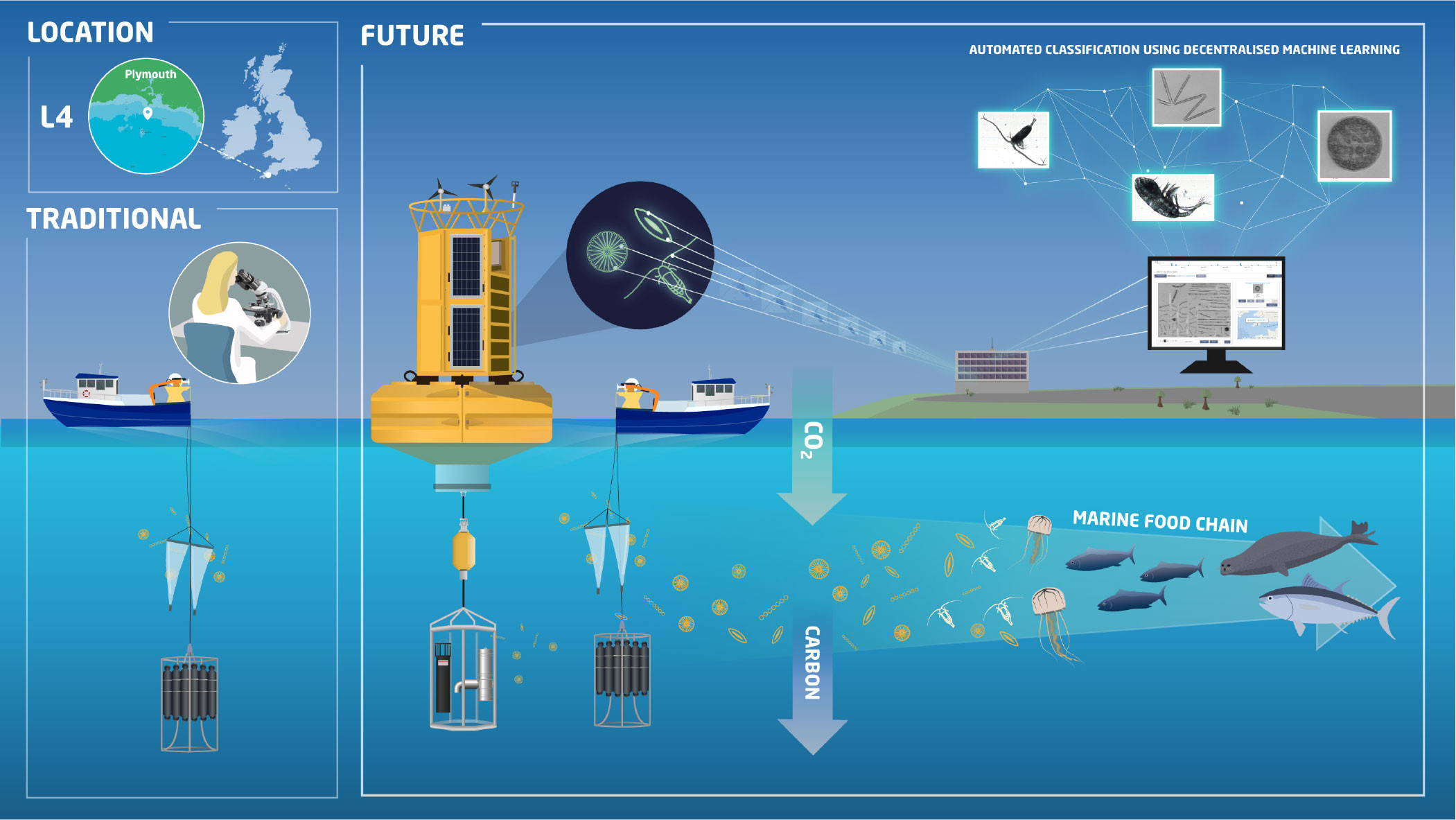
Traditional plankton sampling techniques include net hauls using standard mesh sizes and water sample collection using Niskin bottles. Samples are preserved and taxonomically identified and counted in the laboratory using light microscopy. This process has several drawbacks. For example, standard mesh sizes can be biased toward certain plankton sizes, and fragile or gelatinous organisms can be damaged or fragmented during the sampling and preservation process, making identification difficult. Further, there is no opportunity to observe behavioral interactions among organisms (Remsen et al., 2004; Greer et al., 2021).
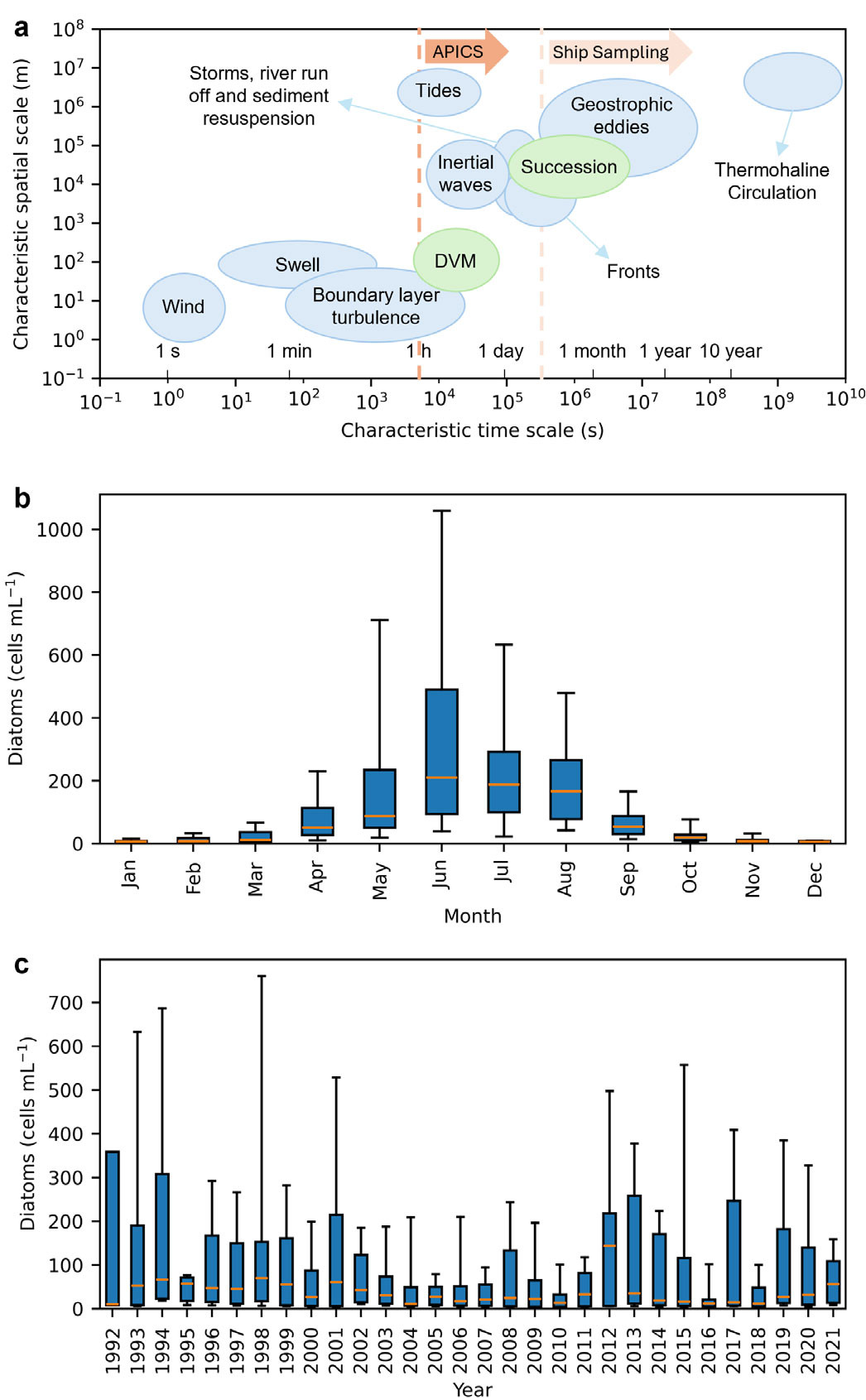
Ship-based sampling is also time-consuming and restricted to periods when there is good weather, limiting the frequency at which data can be collected. Historically, plankton samples from Station L4 have been collected once a week (weather permitting), enabling important seasonal and interannual changes to be quantified and studied. However, numerous processes that influence plankton population numbers and community composition have shorter characteristic timescales and thus are either missed or aliased by weekly sampling. These include successional changes in the plankton community that characterize bloom events or the recovery of the community following passage of a storm (Topor et al., 2022), diel vertical migrations (DVMs) that are undertaken by many larger zooplankton (Parra et al., 2019), and changes due to the tide (Figure 1). Fine-scale spatial heterogeneity, for example, caused by the presence of fronts, can also result in large apparent changes in community composition as water masses hosting different communities are advected over the sampling site. Collectively, these processes complicate the interpretation of weekly time series.
|
|
In an attempt to address these issues, several groups around the world have now pioneered the use of underwater cameras to monitor plankton communities (Lombard et al., 2019). Building on these efforts, a new WCO Automated in situ Plankton Imaging and Classification System (APICS) is being operationalized. Distinguishing features of the system include (1) the use of two autonomous submersible camera units—an Imaging FlowCytobot (IFCB) and a Moonpool Plankton Imager (PI-10)—that provide broad size spectrum imaging capability, and (2) a configuration that facilitates deployment under a remotely operated, moored buoy (Figure 2). The system will image both phytoplankton and zooplankton (size range <10 µm–20 mm) at a depth of 10 m. APICS will enable a ~100-fold improvement in sampling frequency, which allows many of the processes missed by weekly sampling to be resolved (Figure 1a). The cameras sample a known volume of water, with the IFCB imaging a 5 mL sample every 20 minutes and the PI-10 actively pumping water through the camera unit at a rate of 34 L min–1 to ensure organisms present at lower concentrations (~100 individuals per m3) can be accurately enumerated. Real-time data processing will be facilitated using swarm learning (Warnat-Herresthal et al., 2021)—a machine learning methodology that allows multiple owners of biological image data to participate in decentralized, collaborative networks where they can leverage the data and expertise of external partners to obtain better, higher efficacy classification results (Figure 2).
|
|
The WCO’s APICS and similar systems offer advantages over traditional sampling methods for both assessing candidate MPAs and monitoring existing ones. Cameras deployed in situ facilitate the collection of high frequency data over an extended time period, allowing a more detailed picture of the environment to be assembled. In general, it is financially and logistically impossible to collect a similar volume of data using traditional, ship-based sampling methods. Further, the real-time processing of data from autonomous camera systems directly facilitates operational monitoring, including for harmful algal blooms and invasive species, which in turn facilitates more rapid decision-making by marine managers.
Within the WCO, APICS will complement the traditional sampling methods that support the current multiyear time series by allowing short-period processes and controls on biodiversity to be studied and understood. In addition, the continuation and expansion of long-term observations based on traditional methods will remain paramount for studying the long-term impacts of climate change.
ACKNOWLEDGMENTS
The authors acknowledge the Natural Environmental Research Council (UK) for funding APICS (NE/X006018/1) and the WCO time series (NE/R015953/1). The team thanks PML’s Smart Sound Plymouth technical support team and the crew of R/V Plymouth Quest for supporting both projects. We thank Jonathon White for assistance in creating graphics used in this article.