INTRODUCTION
The physiological requirements of marine photosynthesizers dictate their reliance on a slew of nutrients that they must draw from seawater. In addition to the macronutrients nitrogen, phosphorus, and (in some cases) silicon, marine phytoplankton require metal micronutrients in order to photosynthesize, efficiently fix carbon, protect themselves from oxidative stress, or access certain macronutrient pools (Fraústo da Silva and Williams, 2001; Morel et al., 2014). The nutritional status and ecological composition of marine phytoplankton communities thus depend on what might be termed the “nutrientscape” of the sunlit surface ocean in which they live—i.e., the distributions and relative abundances of vital dissolved macro- and micronutrients (e.g., Dutkiewicz et al., 2009). Simultaneously, these distributions are shaped by how surface biological uptake and downward particulate export of nutrients interact with ocean circulation to cycle and transport them through the global ocean (e.g., Sarmiento et al., 2007; Sunda, 2012).
A process key to continued surface-ocean primary productivity is the replenishment of nutrients, lost from the upper ocean through the sinking of biogenic particles, by the upwelling of deep, nutrient-rich waters, which happens primarily in the Southern Ocean surrounding Antarctica (Toggweiler, 1994; Marshall and Speer, 2012; Talley, 2013). In addition, mode waters formed at the northern edge of the Southern Ocean ventilate the thermocline of the low-latitude ocean (Morrison et al., 2022), and the abundance and stoichiometry of nutrients in these waters provide a boundary condition for the supply of nutrients to low-latitude ecosystems by coastal or equatorial upwelling (Sarmiento et al., 2004).
As a body of work beginning two decades ago has shown, the large-scale distributions of dissolved macronutrients—and the systematic differences between these distributions—are largely determined by how biogeochemical cycling of nutrients in the surface Southern Ocean, and its interaction with physical processes, modulates the nutrient content of waters between the zones of deep-water upwelling and thermocline ventilation (Sarmiento et al., 2004, 2007; Weber and Deutsch, 2010; Holzer and Primeau, 2013; Holzer et al., 2014). Since ground-breaking studies in the 1970s, it has also been known that vertical profiles of the concentrations of the metals zinc (Zn), cadmium (Cd), and nickel (Ni) in seawater mimic those of the major nutrients (Figure 1; Boyle et al., 1976; Sclater et al., 1976; Bruland et al., 1978). By vastly expanding data coverage and providing basin-scale sections of the abundance and stable isotope composition of these micronutrient metals, the GEOTRACES program has allowed a reevaluation of the mechanisms responsible for this striking similarity.
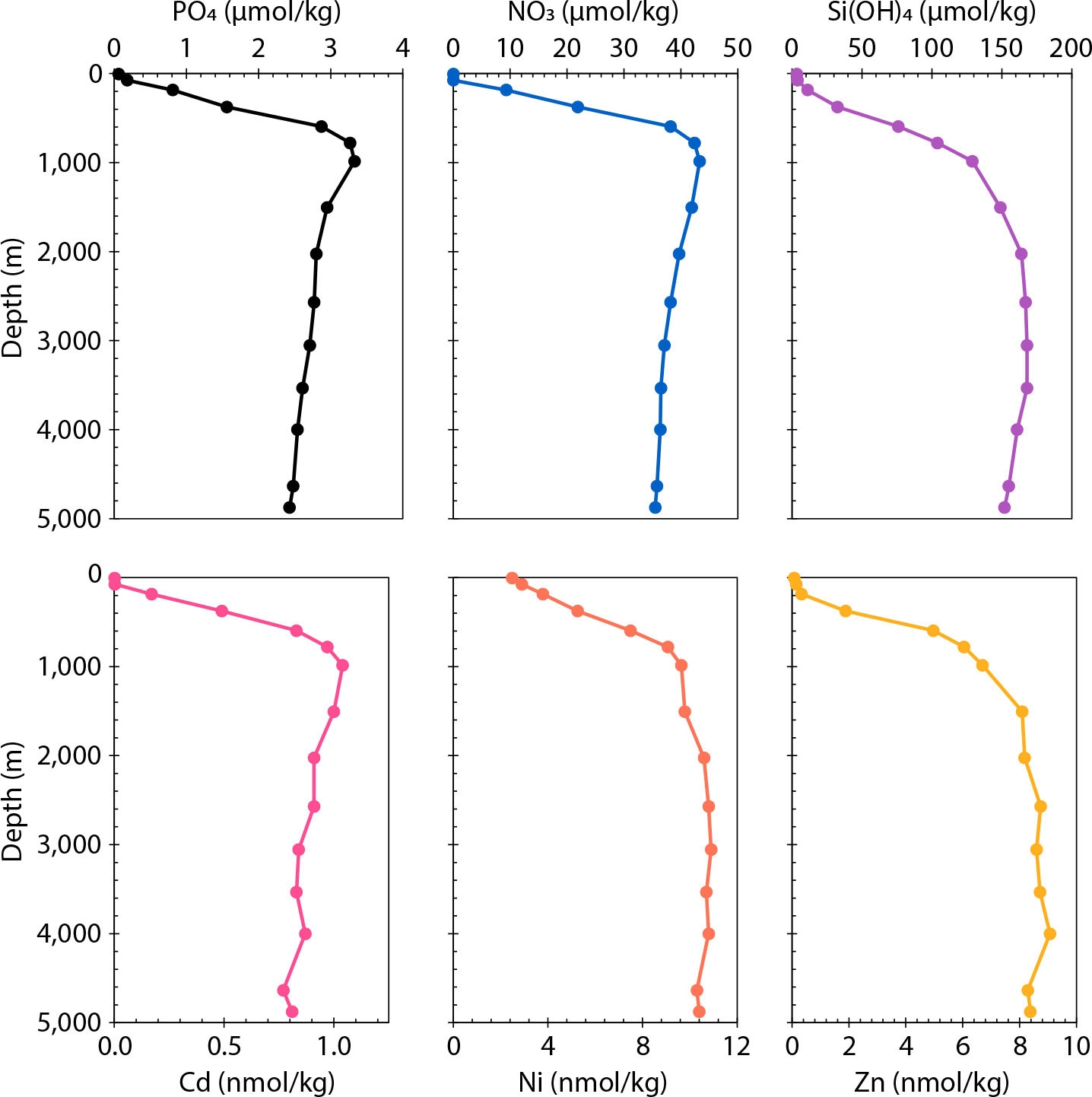
FIGURE 1. Profiles of the macronutrients (upper row) and metal micronutrients (lower row) from the subtropical North Pacific (32.7°N, 145°W) are plotted as reported by Bruland (1980). > High res figure
|
Micronutrient Mimics
Zinc, Cd, and Ni are only a few of the micronutrients that phytoplankton need; we focus on them here for reasons both conceptual and practical. Conceptually, the similarities and differences between their elemental and isotopic distributions nicely illustrate how Southern Ocean processes can shape global nutrient distributions. Practically, they each have a stable isotope system that provides an additional constraint on their cycling, and there are sufficient data to characterize their behavior in the Southern Ocean. We thus only very briefly consider the vital micronutrients iron and manganese, whose global distributions are not affected by Southern Ocean processes (see Box 1), and do not discuss the micronutrients cobalt, copper, and selenium, which are not controlled by the Southern Ocean or for which Southern Ocean data coverage remains too sparse.
Zinc, Cd, and Ni all display what have classically been called “nutrient-type” distributions (Bruland, 1983): that is, their dissolved concentrations are at a minimum in the surface mixed layer and increase both downward in the water column and in deep waters from the North Atlantic to the North Pacific—thus mimicking the distributions of the macronutrients. This similarity can be seen in the vertical profiles of Figure 1, which also reveal differences between the three metals: the distribution of dissolved Cd most closely resembles those of nitrate (NO3) and phosphate (PO4), exhibiting the same increase through the thermocline and mid-depth maximum as these major nutrients (Boyle et al., 1976). Zinc, on the other hand, bears more similarity to silicic acid (Si(OH)4), with low concentrations extending from the surface ocean deeper into the thermocline, and reaching a maximum closer to the base of the water column (Bruland et al., 1978). A particularity of the Ni distribution (Sclater et al., 1976) is that, unlike the macronutrients or Zn and Cd, its concentration in the surface ocean never decreases below about ~20% of deep-water values, even in the nutrient-poor subtropical gyres (in contrast to <1% for Zn and Cd).
These three elements also have differing roles in phytoplankton biochemistry. Zinc has the most diverse set of biochemical roles in photosynthesizers, including as the metal center in the enzyme carbonic anhydrase, important for the efficient fixation of carbon (Morel et al., 1994, 2014). When ambient Zn concentrations are low, many phytoplankton can substitute cobalt or Cd for it (Lee and Morel, 1995; Sunda and Huntsman, 1995). Such “cambialistic” substitution for Zn may be the primary reason that Cd behaves as a micronutrient in the sea, because its only known biochemical role is in a Cd-bearing carbonic anhydrase in some marine diatoms (Lane et al., 2005). In phytoplankton, Ni is mainly used in nitrogen metabolism or to protect from oxidative stress (Ragsdale, 2009): it is associated with the metalloenzyme urease, which catalyzes the metabolism of urea to ammonia; NiFe hydrogenase, which prevents the inhibition of nitrogen fixation by O2 and H2 in diazotrophs; and a Ni-bearing superoxide dismutase primarily found in cyanobacteria, which gets rid of harmful reactive oxygen species.
At the time when the first analyses of these micronutrients in seawater were made, the marine biogeochemical paradigm interpreted major nutrient distributions in an essentially one-dimensional fashion (e.g., Broecker and Peng, 1982). In that view, concentrations of silicic acid increase deeper in the water column than the other two macronutrients (Figure 1) because siliceous hard parts dissolve more slowly than bacteria decompose organic matter—so that Si is released from sinking particles at greater depths than NO3 and PO4. The similarities between the distributions of Zn, Cd, and Ni and the macronutrients were analogously interpreted as resulting from directly coupled cycling: for instance, that profiles of Zn and Si look similar because Zn is incorporated into the siliceous frustules of diatoms (Bruland et al., 1978). But we now know that the marine distributions of dissolved nutrients are produced by a complex set of three-dimensional interactions between biologically driven fluxes and physical transport and mixing processes. On both these fronts, Southern Ocean processes play a key role at the scale of the global ocean.
THE SOUTHERN OCEAN CIRCULATION HUB
The current paradigm for explaining Southern Ocean nutrient resupply emphasizes a longitudinally averaged, two-dimensional framework with the meridional overturning circulation as the primary driver of upward nutrient transport (e.g., Sarmiento et al., 2004; Morrison et al., 2015). The upwelling limb of the overturning circulation is largely controlled by the westerly winds over the Southern Ocean, which drive northward Ekman transport in the surface layer. South of the latitude where the westerly winds are strongest (~50°S), the Ekman transport is divergent and thus draws up nutrient-rich deep waters from below, along the sloping density layers of the Southern Ocean. Upon reaching the surface mixed layer, one portion of these upwelled waters flows northward in the “upper” overturning cell, while the other portion flows south toward Antarctica in the “lower” overturning cell. It is the upper overturning cell that dominates the supply of nutrients for global primary production, due to its northward surface flow (Marinov et al., 2006; Primeau et al., 2013). The upwelling waters of the lower overturning cell have limited residence time when they outcrop at the surface around Antarctica, as they are rapidly returned to the abyss, influencing deep water nutrient distributions (Sarmiento et al., 2007).
Recent work has highlighted the unique three-dimensional structure of Southern Ocean upwelling. Rather than being longitudinally homogeneous, the upwelling is intensified in a handful of eddy hotspots on the eastern side of large bathymetric features (Figure 2a; e.g., Foppert et al., 2017; Tamsitt et al., 2017). The localization of the upwelling is particularly prominent at ~1,000 m depth (Tamsitt et al., 2017; Yung et al., 2022), but localization is also evident at the base of the mixed layer (Viglione and Thompson, 2016). Modeling studies suggest that the net circumpolar upwelling transport of nutrients along isopycnals is similarly dominated by eddies at a small number of localized hotspots (Dufour et al., 2015). In addition to this intensified upwelling transport at eddy hotspots, there may also be significant larger-scale inter-basin differences in upwelling transport of deep waters into the mixed layer (Viglione and Thompson, 2016; Prend et al., 2022). However, an open question remains regarding what impact any longitudinal variations in nutrient delivery to the mixed layer have on the distribution of nutrients and phytoplankton ecology across the Southern Ocean. The distribution of biogeochemical properties in the surface Southern Ocean is strongly guided by the Antarctic Circumpolar Current (ACC), whose flow is organized into a series of jets aligned with strong density fronts (i.e., sharp changes in the physical structure of the water column on either side of the jet). Nutrient concentrations in the surface Southern Ocean are generally homogeneous between these fronts, with the strongest concentration gradients coinciding with the physical front (Pollard et al., 2002). It may be that, despite inhomogeneous upwelling, the strong flow of the ACC, as well as lateral mixing by eddies, smooths out longitudinal (along-front) gradients in nutrient concentrations (e.g., Morrison et al., 2022).
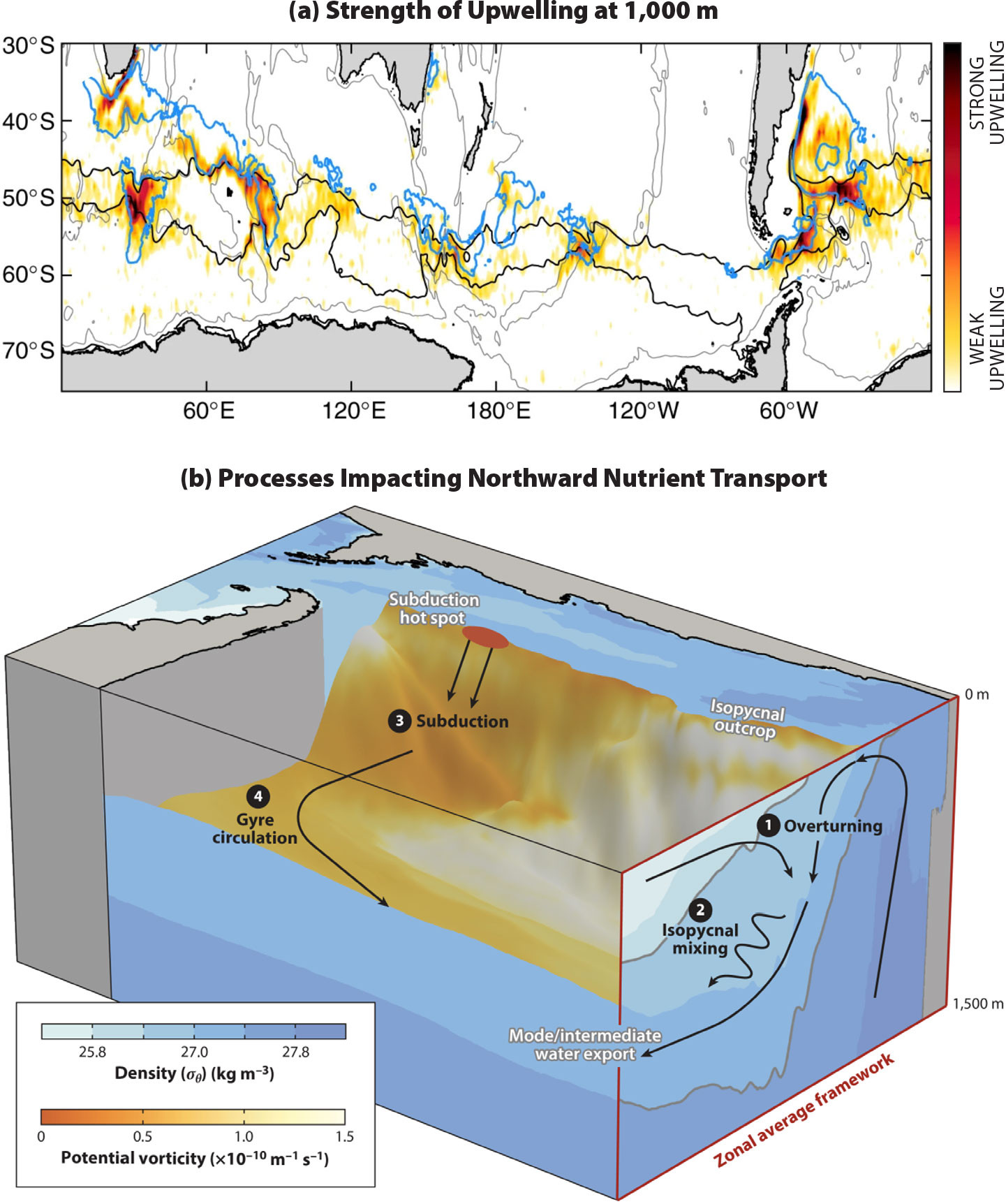
FIGURE 2. The three-dimensional structure of the physical processes impacting nutrients in the Southern Ocean. (a) The inhomogeneous spatial distribution of simulated upwelling of deep waters at 1,000 m depth. Reproduced from Tamsitt et al. (2017) (b) Processes (enumerated) contributing to the export of nutrients northwards into the global thermocline. Blue colors show potential density, and orange colors show potential vorticity on a density surface in the interior. Low potential vorticity is an indicator of recently ventilated mode water. Reproduced from Morrison et al. (2022). > High res figure
|
The mode waters of the global thermocline are replenished and enriched by subduction on the northern edge of the Antarctic Circumpolar Current (Sallée et al., 2010). The subducting waters are a mixture of both northward-flowing Southern Ocean surface waters and subtropical waters flowing southward in western boundary currents (Figure 2b). While many nutrients exhibit negligible concentrations in the subtropical source waters, it is still necessary to consider contributions from both northern and southern source waters to understand nutrient distributions in the mode waters (Fernández Castro et al., 2022). Many conceptual frameworks and schematics of mode water formation focus exclusively on the role of the two-dimensional longitudinally averaged overturning circulation (e.g., Sarmiento et al., 2004; Marinov et al., 2006). However, like the upwelling, the transfer of nutrients from the surface of the Southern Ocean northward into the global thermocline is a complex, three-dimensional process (Morrison et al., 2022). Subduction of waters from the mixed layer into the interior occurs in localized hotspots, with substantial inter-basin differences (Sallée et al., 2010). After subduction, water mass properties are rapidly homogenized across the mode waters by eddy mixing and gyre circulation (e.g., Gupta et al., 2022). Eddy-driven mixing along isopycnals may also contribute substantially to the northward flux of nutrients into the mode waters, depending on the nutrient gradient along isopycnals (Fripiat et al., 2021).
THE SOUTHERN OCEAN NUTRIENT HUB
The role of the Southern Ocean in both the upwelling of deep waters and the ventilation of the low-latitude thermocline makes it an important “hub” of the global overturning circulation. As a result, the physical and biogeochemical properties of abyssal, intermediate, and upper-ocean water masses formed here influence tracer distributions at a near-global scale. When it comes to the distributions of macro- and micronutrients, what is particularly important is how the uptake of nutrients by Southern Ocean ecosystems modifies the properties of nutrient-rich waters upwelled to the surface, before they are subducted into the ocean interior as mode and intermediate waters.
This is shown most clearly by the effect of Southern Ocean nutrient uptake on macronutrients (Sarmiento et al., 2004, 2007). Primary production in the high-latitude Southern Ocean is dominated by diatoms—phytoplankton with opaline cell walls that thrive in dynamic, highly seasonal and competitive environments (Margalef, 1978). Probably as the combined result of limitation by light and Fe (see Box 1) and adaptation to Si-rich waters fed by deep-water upwelling, Southern Ocean diatoms are heavily silicified (Baines et al., 2010). They thus draw down Si much more strongly than the other major nutrients NO3 and PO4, so that as surface waters spiral northward across the fronts of the ACC, Si is depleted more quickly (i.e., further south) than nitrate or phosphate (Figure 3). Surface ocean Si concentrations are generally depleted north of the Polar Front, the southernmost of the ACC fronts north of the upwelling zone (Pollard et al., 2002).
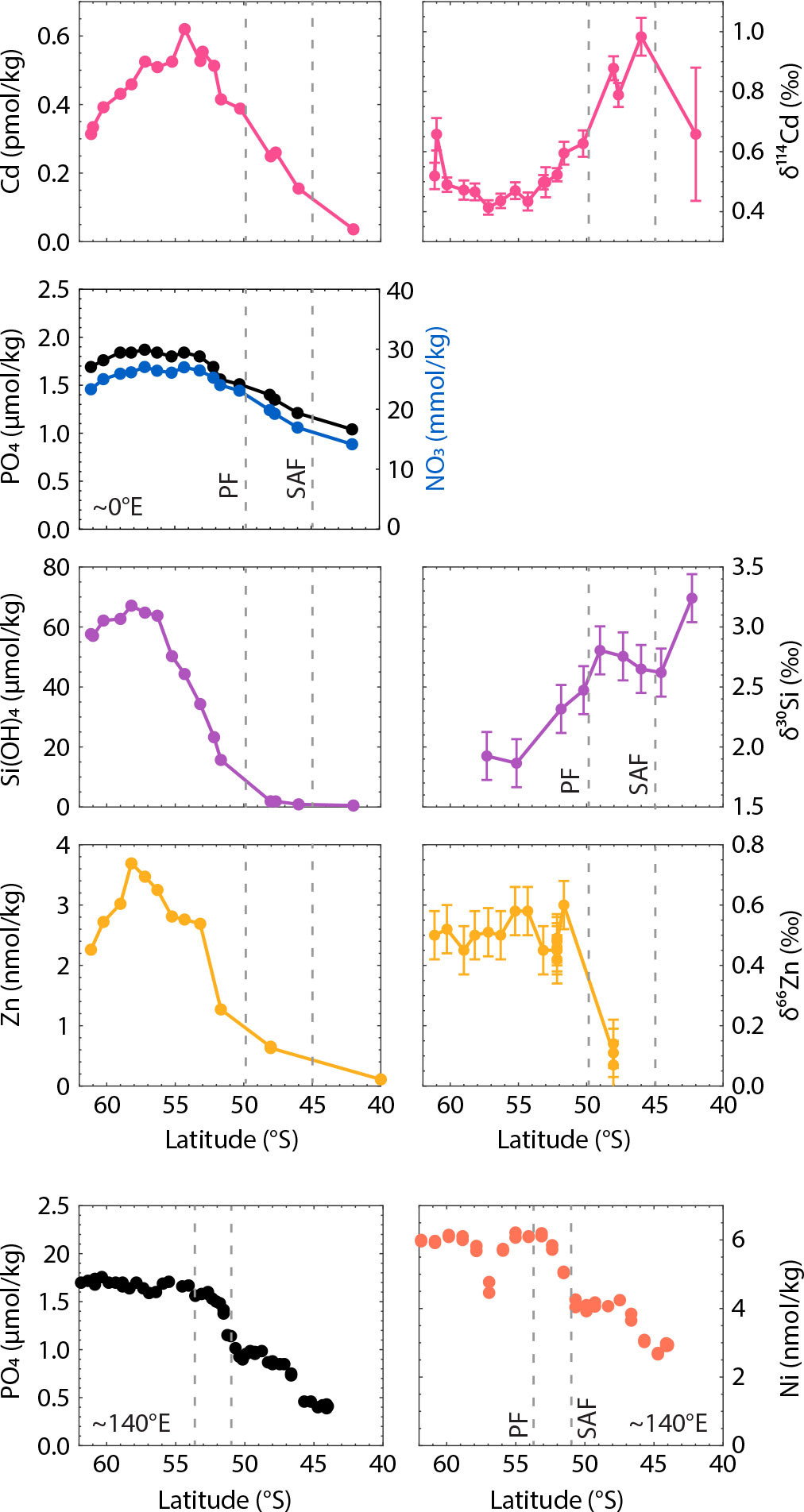
FIGURE 3. Latitudinal variation in macro- and micronutrient concentrations and isotopic compositions in the surface Southern Ocean. The upper four rows show data from the GEOTRACES GIPY04/GIPY05 transect mostly along the zero meridian (Abouchami et al., 2011; Fripiat et al., 2011; Zhao et al., 2014; Wyatt et al., 2014). No δ15N-NO3 data are available for this transect; see Sigman et al. (1999) for a Pacific-sector cross-frontal δ15N-NO3 section. The bottom row shows data from the surface ocean (<25 m) from GEOTRACES GIPY06 transect along ~140°W. These data are unpublished and reproduced with kind permission of Andrew Bowie. PF and SAF denote approximate positions of the Polar Front and Subantarctic Front. Error bars on isotopic data represent uncertainty as reported by the authors. > High res figure
|
The mode and intermediate waters formed at the northern edge of the ACC inherit this surface signal and are thus relatively poor in Si—a characteristic that they impart to the low-latitude thermocline. Thus, the fact that Si concentrations in the upper ocean are low relative to nitrate and phosphate (Figure 1), and increase deeper in the thermocline, has less to do with the fact that dissolution of (Si-bearing) opal takes place deeper than the remineralization of (N- and P-bearing) organic matter; rather, it stems from the fact that high-latitude diatoms have more efficiently stripped Si out of the source regions of the waters that ventilate the thermocline (Sarmiento et al., 2004, 2007; Holzer et al., 2014).
Macronutrient Isotopes and the Global Reach of Southern Ocean Uptake
This southerly view of global marine nutrient cycling emerged shortly before the first GEOTRACES expeditions during the International Polar Year 2007–2008, and has been substantiated by macronutrient stable isotope data that emerged partly from those early Southern Ocean transects. Phosphorus has only one stable isotope, but the stable isotope compositions of seawater nitrate and silicic acid are expressed using the δ notation, which represents the deviation (in parts per thousand) of the isotope ratio 15N/14N or 30Si/28Si from that of a standard, for example:
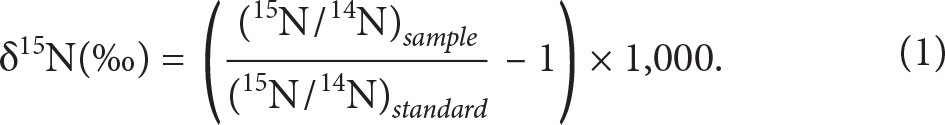
|
An increase in the δ15N of seawater nitrate (δ15N-NO3) or the δ30Si of silicic acid thus reflects an enrichment in the heavier isotopes of these elements. Biological uptake of nitrate and silicic acid is associated with isotope fractionation (Wada and Hattori, 1978; De La Rocha et al., 1997), with preferential uptake of the lighter isotopes leaving the residual dissolved pool isotopically heavy (i.e., with high δ15N-NO3 or δ30Si). Thus, in the Southern Ocean, as the concentrations of nitrate and silicic acid decrease across the fronts of the ACC, δ15N-NO3 and δ30Si increase (Figure 3; Sigman et al., 1999; Varela et al., 2004). These isotopic fingerprints of biological uptake are inherited by mode and intermediate waters when they form at the northern edge of the ACC—thus making nitrate and silicic acid in the thermocline isotopically heavy relative to the deep ocean (Sigman et al., 2000; Fripiat et al., 2011, 2023).
It is the presence of these water masses that causes δ15N-NO3 and δ30Si to increase at intermediate depths (Figure 4) in most of the mid- and low-latitude ocean; indeed, the thermocline distributions of δ15N-NO3 and δ30Si trace the equatorward spreading of mode and intermediate waters within the subtropical gyre circulation (de Souza et al., 2012a; Rafter et al., 2013; Grasse et al., 2020). In the Pacific, the biogeochemical impact of southern-sourced mode and intermediate waters is mostly restricted to the Southern Hemisphere (e.g., Sarmiento et al., 2004), but in the Atlantic Ocean, elevated δ15N-NO3 and δ30Si signals extend all the way to the North Atlantic and Arctic Oceans. This is because the upper limb of the overturning circulation transports southern-sourced waters into the Northern Hemisphere, eventually feeding the formation of North Atlantic Deep Water (Talley, 2013). Nitrate and silicic acid in North Atlantic Deep Water, whose constituent water masses form in the subpolar North Atlantic and Arctic, thus bear the telltale elevated δ15N-NO3 and δ30Si values that result from biological uptake at the other end of the globe, around Antarctica (de Souza et al., 2012b, 2015; Brzezinski and Jones, 2015; Holzer and Brzezinski, 2015; Marconi et al., 2015; Varela et al., 2016).
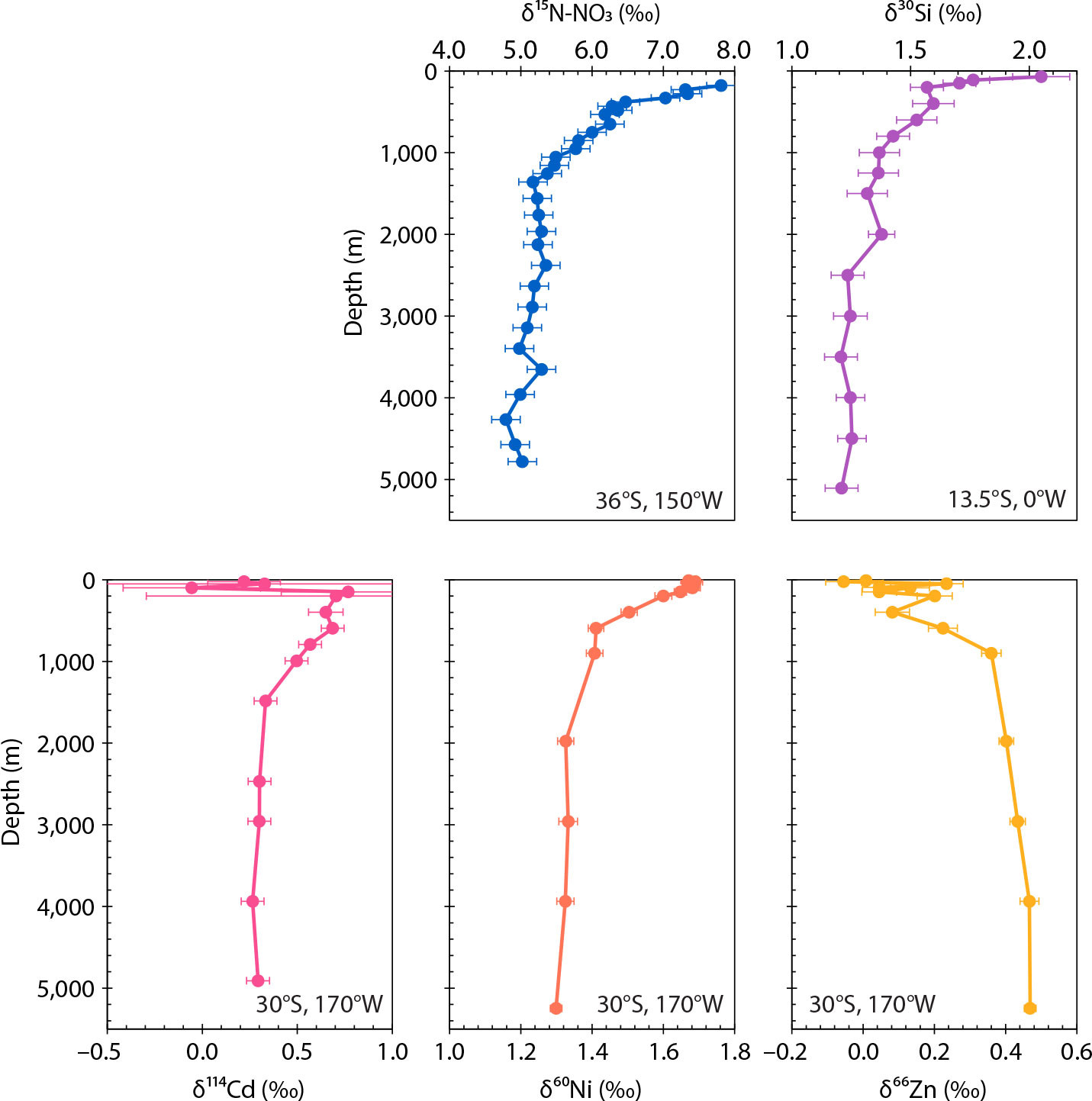
FIGURE 4. Profiles of the isotopic composition of macronutrients (upper row) and micronutrients (lower row) in the southern (sub)tropics. Micronutrient isotope data are all from the same station (GR15) occupied during GEOTRACES section GP19 in the southwest Pacific (Takano et al., 2017; Sieber et al., 2019; see also Figure 5). Macronutrient isotope data are from de Souza et al. (2012b) and Rafter et al. (2013). Error bars show uncertainty as reported by the authors. > High res figure
|
From Macronutrients to Micronutrients
The Southern Ocean control on large-scale nutrient cycling discussed above is generalizable to the micronutrients: if Southern Ocean ecosystems tend to take up a nutrient in excess of the major nutrients nitrate or phosphate, its large-scale distribution will be skewed toward the deep and abyssal ocean, because efficient export to depth in the Southern Ocean will reduce its concentrations in the thermocline and, ultimately, in deep waters formed at high northern latitudes. By providing datasets of consistently co-sampled micronutrient metals and macronutrients, the GEOTRACES program has enabled the systematic study of their relationships in the surface Southern Ocean, while basin-scale sections of metal concentrations and isotopes provide larger-scale constraints. Next, we review the progress made in understanding the role of the Southern Ocean in the marine Zn, Cd, and Ni cycles in the GEOTRACES era.
Zinc and Its Isotopes
Given what we know about how highly silicified Southern Ocean diatoms govern the marine Si distribution, it might be thought that the similarity between Zn and Si comes about because these diatoms also incorporate a lot of Zn into their siliceous frustules (Bruland et al., 1978). But because of its importance in the biochemistry of these eukaryotes, most of their cellular Zn is situated in their organic matter, with at most a few percent in the frustule (Ellwood and Hunter, 2000; Twining et al., 2014; although see the recent results of Grun et al., 2023). Why, then, does the marine Zn distribution not look more like that of NO3 and PO4, which are released back to seawater when organic matter is remineralized? The answer lies in the fact that phytoplankton trace metal quotas exhibit considerable variability at both the phenotypic and physiological levels (e.g., Ho et al., 2003; Twining and Baines, 2013). So, on the one hand, eukaryotic phytoplankton have generally higher cellular Zn quotas than prokaryotes (Saito et al., 2003), and diatoms have even higher Zn quotas than other co-existing eukaryotes. On the other hand, laboratory cultures have shown that the Zn quotas of diatoms and other eukaryotic phytoplankton increase when they are growth-limited by Fe (Sunda and Huntsman, 2000) or when ambient seawater has higher concentrations of the free Zn ion (see Box 2; Sunda and Huntsman, 1992).
So, when deep-water upwelling in the Southern Ocean supplies plenty of Zn to Fe-limited diatom communities, the ecosystem response is to take up large amounts of Zn: diatoms growing in Zn-rich waters south of the Antarctic Polar Front have Zn:P ratios that can be more than 10 times those of low-latitude phytoplankton (Twining and Baines, 2013). As a result, Zn drawdown across the fronts of the ACC is as strong as that of Si, with Zn concentrations decreasing by a factor of 3 across the Antarctic Polar Front and becoming essentially depleted, together with Si, in the formation regions of mode and intermediate waters further north (Figure 3, Ellwood, 2004, 2008; Zhao et al., 2014; Janssen et al., 2020). Thus, although there is no biochemical coupling between their uptakes, the fact that Southern Ocean ecosystems strip both Si and Zn from surface waters means that Zn is “trapped” within the Southern Ocean like Si, rather than being exported into the low-latitude thermocline like NO3 and PO4 (Vance et al., 2017; de Souza et al., 2018). While this leading-order role of Southern Ocean uptake in governing the marine Zn distribution is clear from numerous studies (e.g., Roshan et al., 2018; Weber et al., 2018; Middag et al., 2019), it has been proposed that additional processes involving the reversible sorption of Zn to sinking particles must work north of the Southern Ocean, in order to maintain the similarity between the Zn and Si distributions even in regions not dominated by Southern Ocean water masses, such as the North Pacific (John and Conway, 2014; Weber et al., 2018; Zheng et al., 2021; Sieber et al., 2023a).
The most intriguing aspect of marine Zn is its stable isotope composition δ66Zn, which has a seawater distribution unique among the nutrient isotope systems. Even though most phytoplankton have been shown to preferentially take up the light isotopes of Zn in laboratory culture (e.g., John et al., 2007; Samanta et al., 2018; Köbberich and Vance, 2019), seawater δ66Zn is low in the thermocline and surface ocean, rather than high as for the macronutrients (Figure 4; Conway and John, 2014; Takano et al., 2017; John et al., 2018). This is certainly not the result of Southern Ocean isotope fractionation: here, as surface Zn concentrations drop precipitously toward the north, δ66Zn values show a minimal but just-resolvable increase (Zhao et al., 2014; Wang et al., 2019; Sieber et al., 2020). But this slight signal of biological fractionation appears to be lost north of the Subantarctic Front (Figure 3; Zhao et al., 2014; Ellwood et al., 2020; Sieber et al., 2020), perhaps because of the admixture of isotopically light zinc carried southward in subtropical thermocline waters (Figure 2b; Takano et al., 2017; Samanta et al., 2017). What exact processes are responsible for low subtropical δ66Zn is currently under debate, with two contrasting hypotheses suggested: (1) the removal of isotopically heavy Zn sorbed to sinking particles (John and Conway, 2014; Weber et al., 2018; Sieber et al., 2023a), which requires chelation by natural organic ligands (see Box 2) to prefer isotopically light Zn, at odds with laboratory studies of organic chelators (e.g., Ban et al., 2002; Marković et al., 2017); or (2) the addition of isotopically light Zn from external sources that may be anthropogenic (Lemaitre et al., 2020; Liao et al., 2020), although analyses of open-marine aerosols (Dong et al., 2013; Packman et al., 2022; Zhang et al., 2024) have thus far not revealed Zn isotope compositions that can explain the lowest δ66Zn values observed in thermocline waters. Whether either, both, or neither of these hypotheses explains the thermocline δ66Zn distribution remains the subject of active research.
Cadmium and Its Isotopes
Dissolved and particulate marine data consistently show that, of all the trace metals, Cd behaves most similarly to the macronutrients, being cycled tightly together with P (e.g., Abouchami et al., 2011; Twining et al., 2015; Yang et al., 2018; Middag et al., 2018; Ohnemus et al., 2019; Cloete et al., 2021). Its isotopic behavior, too, is most like the macronutrient systems δ15N-NO3 and δ30Si. Biological uptake fractionates the isotopes of Cd, and as the concentrations of Cd decrease across the fronts of the ACC, it becomes increasingly enriched in the heavy isotopes (i.e., δ114Cd increases; Figure 3; Abouchami et al., 2011; Xue et al., 2013). This similarity to the macronutrient stable isotope systems extends to much of the global ocean: as with δ15N-NO3 and δ30Si, Cd subducted in the mode and intermediate waters is isotopically heavy (Figure 4; Abouchami et al., 2014; Sieber et al., 2019a), and this elevated δ114Cd signal can be traced through the subtropical thermocline (Conway and John, 2015; Xie et al., 2017; George et al., 2019; Sieber et al., 2019b, 2023b) all the way into North Atlantic Deep Water (Abouchami et al., 2014; Conway and John, 2015).
But given its limited biochemical role, why should phytoplankton uptake of Cd so exactly mimic phosphate? In fact, in detail it does not: Southern Ocean surface transects show that Cd is taken up more strongly relative to PO4, with Cd reaching low levels north of the Subantarctic Front, while PO4 remains undepleted (Figure 3; Ellwood, 2008; Abouchami et al., 2011; Baars et al., 2014). This is consistent with two independent sets of observations. First, particulate data show that biogenic particles in the Southern Ocean are highly enriched in Cd relative to P (Bourne et al., 2018; Twining and Baines, 2013). Second, cultures of eukaryotic phytoplankton and incubations of natural assemblages have shown that they will take up more Cd under conditions found in the surface Southern Ocean: under growth limitation by Fe (Cullen et al., 2003), at high concentrations of the free Cd ion (see Box 2; Lee et al., 1995; Sunda and Huntsman, 1998), or at low concentrations of free Mn or Zn (see Box 1; Cullen et al., 1999; Sunda and Huntsman, 2000). So, Fe- and/or Mn-limited Southern Ocean diatom communities would be expected to take up more Cd even south of the Polar Front, and Cd uptake should increase even more strongly when Zn is reduced to low levels to its north, as is indeed observed (Figure 3; Sunda and Huntsman, 2000; Ellwood, 2008).
The stronger Southern Ocean uptake of Cd has also helped to clarify a feature of its distribution that long perplexed oceanographers: the low Cd:PO4 ratio of North Atlantic Deep Water that results in a slight nonlinearity (known affectionately as “the kink”) in the Cd–PO4 relationship in the Atlantic Ocean (Boyle, 1988; Frew and Hunter, 1992; de Baar et al., 1994). In the context of the Southern Ocean nutrient hub as it works for Zn and Si, and the influence of uptake on the Cd distribution in the Southern Ocean surface, it has become clear that the relative Cd-poverty of North Atlantic waters comes about because Southern Ocean phytoplankton reduce Cd concentrations in the upper limb of the overturning circulation slightly more than they do PO4 (Baars et al., 2014; Quay et al., 2015; Middag et al., 2018; Roshan and DeVries, 2021), such that Cd is depleted relative to PO4 in the thermocline (Figure 5).
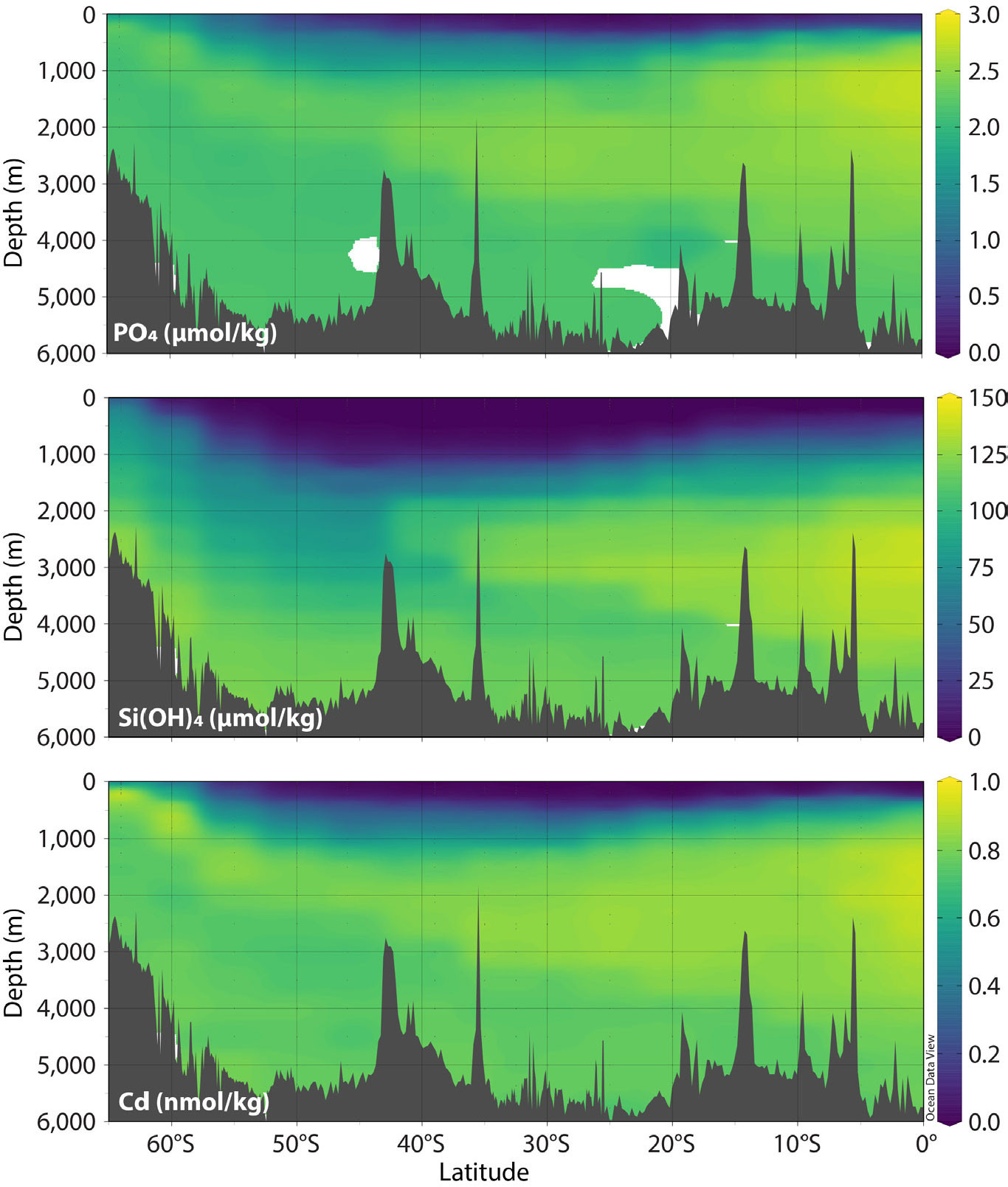
FIGURE 5. Meridional sections of phosphate, silicic acid, and Cd in the southern Pacific from GEOTRACES section GP19. Macronutrient data are from the GEOTRACES IDP2021 (GEOTRACES Intermediate Data Product Group, 2021) and reproduced with kind permission of Toshitaka Gamo and Hajime Obata. Cadmium data are from Sieber et al. (2019). > High res figure
|
Nickel and Its Isotopes
Perhaps because it is never drawn down to very low concentrations in the surface ocean, nickel has until recently received less attention than Zn and Cd, and thus concentration and especially isotopic data are sparser for this metal. Nonetheless, it is clear that across the fronts of the ACC, Ni concentrations decrease much less strongly than those of Zn and Cd. Surface Ni concentrations decrease by just 20% across the Polar Front, and remain above 3 nmol kg–1—~50% of the highest surface Southern Ocean concentrations—even north of the Subantarctic Front, where both Zn and Cd are depleted (Figure 3; Ellwood, 2008; Janssen et al., 2020). In the oligotrophic subtropics, where all inorganic macro- and micronutrients are at very low concentrations, Ni is present at around 2 nmol kg–1, 20% of deep-ocean levels (Figure 1; Middag et al., 2020). It was proposed that this concentration might represent a pool that is not bioavailable (Price and Morel, 1991; Mackey et al., 2002), but recently, nutrient amendments have shown that both eukaryotic and prokaryotic phytoplankton can draw down this pool of seawater Ni significantly when provided with sufficient other nutrients (John et al., 2022). Thus, based on biogeochemical modeling, it has been suggested that the non-zero subtropical Ni minimum instead represents a residual pool of Ni “left over” once other nutrients have been depleted, due to the low Ni requirements of Southern Ocean ecosystems (John et al., 2022).
Certainly, a smaller drawdown of Ni in the Southern Ocean is consistent with its limited role in eukaryote biochemistry. Its only known use in these organisms is in urease, which phytoplankton obligately need when their only nitrogen source is urea (Price and Morel, 1991; Egleston and Morel, 2008), but which may be less important in the nitrate-rich Southern Ocean, where urea is only seasonally an important nitrogen source, mostly in the Subantarctic (e.g., Joubert et al., 2011). Furthermore, sparse seasonally resolved observations also suggest only limited Ni uptake by Southern Ocean ecosystems, with summertime mixed-layer Ni concentrations only ~10% lower than in winter both south and north of the Subantarctic Front (Ellwood, 2008; Cloete et al., 2019). This makes it plausible that the marine Ni distribution, like that of Zn and Cd, is also driven by the stoichiometric requirements of diatom-dominated ecosystems in the Southern Ocean.
At the same time, the stable isotope composition of dissolved Ni (δ60Ni) shows that the subtropics cannot just be a passive receptacle for the Ni leftovers of the high latitudes. Values of δ60Ni show no resolvable variability in the Southern Ocean (Cameron and Vance, 2014; Wang et al., 2019; Archer et al., 2020) or in the subpolar North Atlantic (Lemaitre et al., 2022). In the subtropical ocean, however, δ60Ni increases through the upper thermocline to maxima in the surface (Figure 4; Takano et al., 2017; Archer et al., 2020; Yang et al., 2021; Lemaitre et al., 2022). Lemaitre et al. (2022) argue that this isotopic divide results from Ni cycling processes particular to the oligotrophic subtropical ocean, in which the dearth of inorganic nitrogen sources and the domination of the phytoplankton community by prokaryotes should both tend to increase relative Ni demand (see earlier section on Micronutrient Mimics).
This poses an interesting question that remains unresolved: is the marine Ni distribution driven by low-latitude Ni demand by prokaryotes, or instead by the limited Ni requirement of high-latitude eukaryote-dominated ecosystems? In the latter case, the global Ni distribution is controlled from the south much like those of the macronutrients as well as Zn and Cd (John et al., 2022); in the former, the reduction of Ni concentrations in the Subantarctic, and with it the global Ni distribution, is at least partially driven by subtropical Ni drawdown, and the contribution of subtropical waters to Southern Ocean mode waters (Figure 2b; Fernández Castro et al., 2022). This would make the biogeochemical controls on the marine Ni cycle unique among the elements
considered here.
PERSPECTIVES
Data from the first two decades of GEOTRACES show that the Southern Ocean control on global macronutrient distributions is generalizable to numerous micronutrient metals. Simultaneously, physical oceanographic research over the last decade has increasingly revealed the zonal asymmetry of physical Southern Ocean processes key to biogeochemical tracer transport (Figure 2; Morrison et al., 2022; Gray, 2024)—for instance, that the pathways of upwelling and subduction in the Southern Ocean are concentrated in discrete hotspots in the longitudinal direction (e.g., Sallée et al., 2010; Tamsitt et al., 2017).
It remains unknown to what extent this heterogeneity in the physical system impacts the distribution of biogeochemical tracers. Southern Ocean mode waters exhibit co-variation between biogeochemical and physical properties that is consistent between ocean basins (Bushinsky and Cerovečki, 2023). Observations also show that surface-ocean nutrient concentrations are generally homogeneous in the longitudinal direction between Southern Ocean fronts (Pollard et al., 2002), with distributions instead dominated by strong variations in the latitudinal direction (Figure 3). This suggests that the timescales of lateral eddy-driven mixing (also known as isopycnal diffusion) and transport by the ACC in the along-front direction may be fast enough to smooth out any variability arising from localized hotspots of upwelling and subduction. However, research on the three-dimensional structure of physical–biogeochemical interactions in the Southern Ocean is in its infancy (Gray, 2024). More research is needed to robustly determine whether a two-dimensional (depth–latitude), longitudinally averaged framework is sufficient to understand the cycling of nutrients and micronutrients in the Southern Ocean.
A further recent development is broader recognition of the role that subtropical thermocline waters play in setting the (biogeochemical) properties of Southern Ocean mode waters (Iudicone et al., 2011; Morrison et al., 2022; Fernández Castro et al., 2022). Progress in our understanding of how this contribution impacts macro- and micronutrients may be made by considering biogeochemical edge cases such as the metal chromium (Cr), which has no known biological role (Fraústo da Silva and Williams, 2001) but displays a muted gradient across the fronts of the ACC that could result from mixing of subtropical and subpolar waters (Rickli et al., 2019). South of the ACC, the influence of biogeochemical cycling in the gyres of the Ross and Weddell Seas on the vertical—and, subsequently, larger-scale—redistribution of macro- and micronutrients (e.g., MacGilchrist et al., 2019; Sieber et al., 2020) deserves more focused study.
When it comes to the micronutrients Zn, Cd, and Ni specifically, GEOTRACES data have not only clarified the role of the Southern Ocean hub in their marine cycles, but also raised questions that remain to be answered in the years to come.
For Zn, it is the isotopic distribution that remains most debated: will we be able to resolve whether the low δ66Zn of the low-latitude thermocline is a result of internal biogeochemical cycling or external input? Doing so will require challenging work, in the lab and in the natural environment, to robustly quantify the direction and magnitude of the isotope effect associated with binding to natural seawater ligands, provide observational constraints on the distribution coefficients of Zn sorption to different kinds of marine particles, and better characterize the isotopic composition of atmospheric sources of Zn to the ocean.
The large-scale systematics of Cd and its isotopes are less contentious, but one open question pertains to the role of the tropical ocean, including tropical oxygen-minimum zones, in modifying the Cd–PO4 relationship determined by the Southern Ocean. There appears to be a small preferential loss of seawater Cd in the shallow tropical subsurface (Ohnemus et al., 2017; Guinoiseau et al., 2019; de Souza et al., 2022; Sieber et al., 2023b), but whether this is driven by biology or redox conditions (or both, or neither) is currently unclear; a useful first step would be a more detailed characterization of the associated Cd-rich particulates (Ohnemus et al., 2019). The exact controls on the δ114Cd systematics of the thermocline also remain to be elucidated (Xie et al., 2019), and here isotope-enabled models of ocean biogeochemistry may prove to be useful tools.
Open questions abound when it comes to the marine cycle of nickel. Is its cycle driven primarily by low-latitude prokaryote-dominated ecosystems (Lemaitre et al., 2022) or by high-latitude Southern Ocean uptake as for the Zn and Cd (John et al., 2022)? And is the Southern Ocean Ni gradient driven primarily by regional uptake, or rather by the influence of Ni-poor subtropical waters transported southwards into the Subantarctic? Do prokaryotic and eukaryotic phytoplankton fractionate the isotopes of Ni differently during uptake? What is the affinity of Ni for sorption to various types of marine particles? As work proceeds at sea, in the clean lab, and on computing clusters, answers to these questions and more will emerge.
Acknowledgments
We would like to acknowledge Jorge Sarmiento and the stimulating interdisciplinary group of students and postdocs he brought together, which was the inspiration for this collaboration. We thank Andrew Bowie and the IMAS/ACE CRC trace metal team for the Ni data shown in Figure 3, and Toshitaka Gamo and Hajime Obata for the macronutrient data in Figure 5. GFdS is supported by ETH Zurich. AKM is supported by the Australian Research Council Australian Centre for Excellence in Antarctic Science (SR200100008). We declare no conflicts of interest. The international GEOTRACES program is possible in part thanks to the support from the US National Science Foundation (Grant OCE-2140395) to the Scientific Committee on Oceanic Research (SCOR).




 > High res box
> High res box

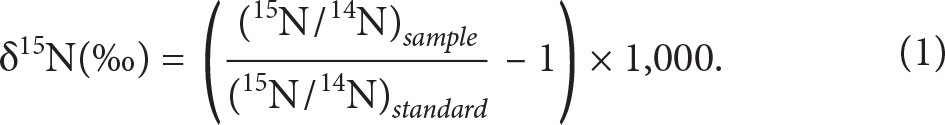

 > High res box
> High res box