INTRODUCTION
The offshore wind (OSW) industry is expanding. Using new technologies, it is projected to install larger capacity devices and arrays in deeper waters and farther from shore (Soares-Ramos et al., 2020). Subsea power cables (hereafter cables) present many potential environmental effects (Taormina et al., 2018). The most topical within the fishing community are the lack of access to fishing grounds due to entanglement risks and the effects of electromagnetic fields (EMFs) on target species. We focus here on EMF emissions in marine environments and their potential influences on the important life processes of resource species (those of commercial or recreational value).
Natural magnetic, electric, and electromagnetic fields provide important ecological cues to magneto-receptive and electro-receptive species. As examples, many species obtain locational and directional cues important for navigation from Earth’s geomagnetic field and associated motionally induced electric fields (Gill et al., 2014), and bioelectric fields help predators detect prey (Bedore and Kajiura, 2013). As natural fields provide cues to identifying and locating resources, distortions of these fields by anthropogenic EMFs may have important ecological consequences.
Sources of OSW cable-related anthropogenic EMFs include (1) inter-array cables between devices (fixed foundation/floating) and substations (Figure 1a,b), and (2) export cables, in varied configurations, transmitting energy to shore (Figure 1c). Cables commonly laid in or on the seabed with protection emit EMFs that can be sensed by benthic species, and floating devices with dynamic cables located in the water column introduce EMFs into the pelagic zone. To date, medium and high voltage alternating current (AC) cables are more common for OSW developments, but direct current (DC) cables have been employed and are advantageous because of their increased capacity and efficacy in longer transmission distances to shore (Soares-Ramos et al., 2020). Project-specific properties will define the cable types and geographical routing through multiple ecosystems, but species biology determines how they perceive EMFs. The present knowledge base has gaps that present challenges for managers of OSW developments and fishery resources; there are no policies or regulations related to EMFs. In a bid to improve our knowledge base to enable better informed management, we briefly review progress to date and identify a path forward.
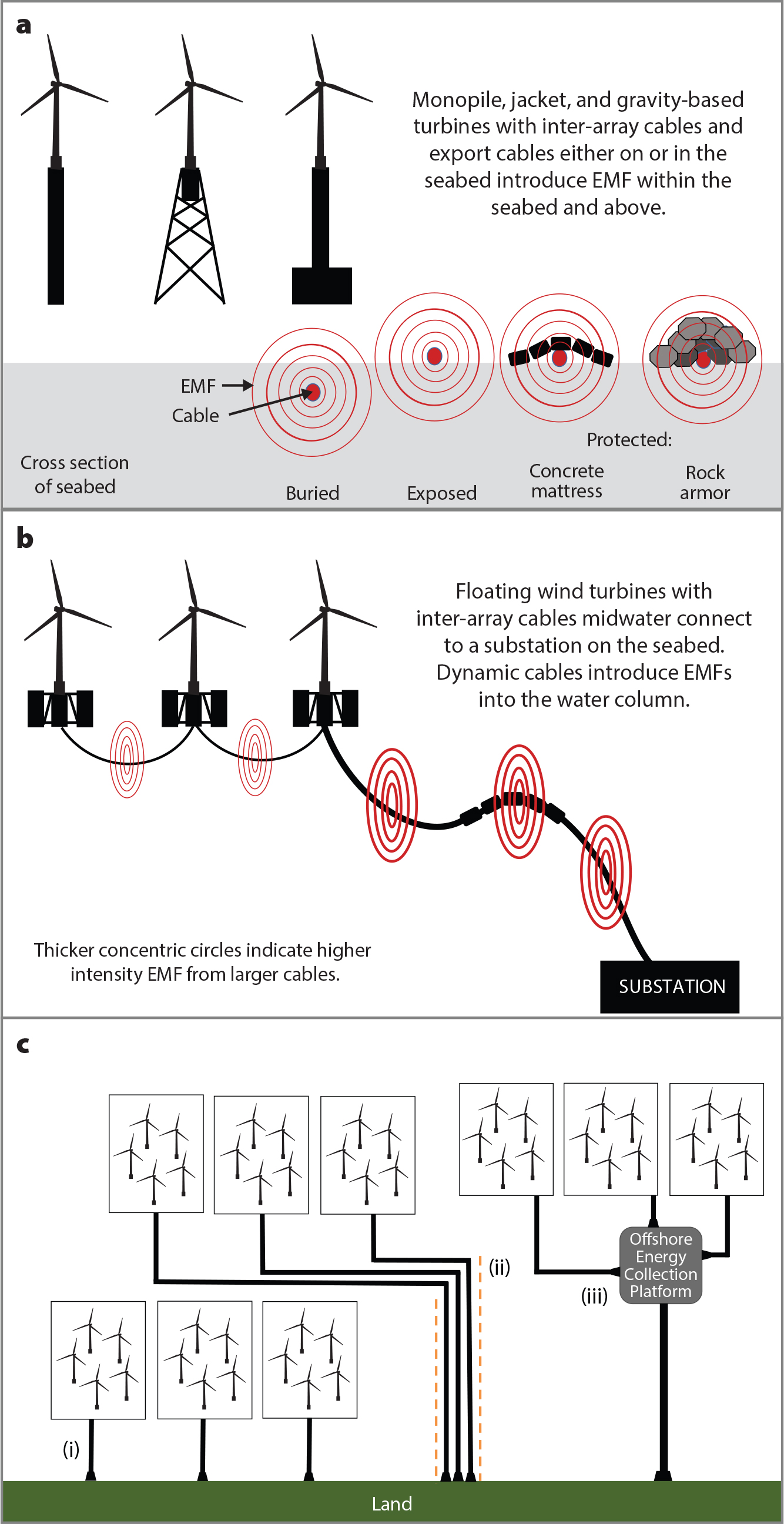
Figure 1. Subsea cables introduce electromagnetic field (EMF) emissions. (a) Benthic EMFs are emitted from export cables and inter-array cables that serve fixed foundation devices, either buried in or laid on the seabed with protection. (b) EMFs are emitted into the pelagic environment from dynamic cables of floating offshore wind (OSW) projects. (c) Cable route configuration options as arrays increase in coastal waters include (i) simple individual exports from each array, (ii) multiple cables that may be in corridors, or (iii) offshore collection platforms that employ higher capacity export cables. > High res figure
|
In this article, we explain the components of EMFs and describe efforts to measure them. Then, taking the vantage point of the receptive species, we consider the layers of information required to enable informed management decisions. By exploring the present knowledge base, we highlight the patchwork of information available from relatively few species obtained through a variety of methods, as well as the need to assess EMF effects amid shifting natural baselines. Although the effects of EMF on resource species is an understudied aspect of OSW environmental impact assessments, we provide a brief synthesis of relevant knowledge of a broader range of species. We then make recommendations on how to address knowledge gaps, with considerations for management needs.
UNDERSTANDING EMF, A POTENTIAL PRESSURE
By definition, EMFs have both magnetic and electric fields. The magnetic field of a cable derives from the movement of electrical current within the cable core. The voltage applied to the cable produces an electric field that is contained within the cable shielding if perfectly grounded (Gill et al., 2012). The magnetic fields are not contained and are emitted into the environment (Figure 1), interacting in three dimensions with the local geomagnetic field (Gill et al., 2014). Due to the rotational nature of the magnetic field associated with AC cables, they also induce electric fields (CMACS, 2003). However, motionally induced electric fields may also arise from an animal or water body moving through the magnetic field produced by both AC and DC cables (Gill et al., 2014).
EMF intensities decay as a function of distance from the source and can be modeled using cable properties (core/shielding materials, configuration, amperage, voltage) and the local geomagnetic field (e.g., Hutchison et al., 2020). Ideally, models should be ground truthed with empirical measurements, but few studies have characterized EMFs in situ at biologically relevant scales. In situ methods have included surface-towed, bottom-towed, and autonomous underwater vehicles, each either measuring the magnetic field or simultaneously measuring magnetic and electric fields in three dimensions (Dhanak et al., 2015; Kavet et al., 2016; Sherwood et al., 2016; Hutchison et al., 2020).
It is important to consider the properties of the cable and the emitted EMF’s three-dimensional interaction with the local geomagnetic field, as well as the spatial extent and intensity of the cable EMF. Regardless of cable capacity, the power transmitted varies temporally and influences the EMF. Furthermore, AC fields emitted over several tens of meters have been measured associated with DC cables (Hutchison et al., 2020), which are presumed to be linked to the AC-DC transformation. Such context is important in determining the exposure and likely species encounter.
An OSW project(s) dictates the number, size, and location of inter-array and export cable(s) to shore. Some European OSW projects have multiple export cables or multicable corridors (Figure 1) that may have interacting EMFs. It is often presumed that cable burial or protections (Figure 1a) reduce EMF effects by increasing the distance from the source, thereby reducing EMF intensity as a function of distance. However, a reduction in EMF intensity may bring the electric and/or magnetic fields into ranges more perceivable by receptive species (Formicki et al., 2019; Newton et al., 2019).
TAKING THE VANTAGE POINT OF THE RECEPTIVE SPECIES
Marine management must draw on the best knowledge available from studying the receptive species and the characteristics that define the pressure; the receptor is central to the scenario (Figure 2a). At the core of understanding how a receptive species perceives EMFs is knowledge of their ability to detect EMFs and their range of sensitivity to them (Figure 2b). However, life stage and movement ecology must also be considered because they inform the likely encounter rate. Collating these information layers provides the fullest picture for management to assess effects. The challenge is to understand how EMFs are perceived by receptive species and which spatiotemporal scales relate to relevant life stages and movement ecology.
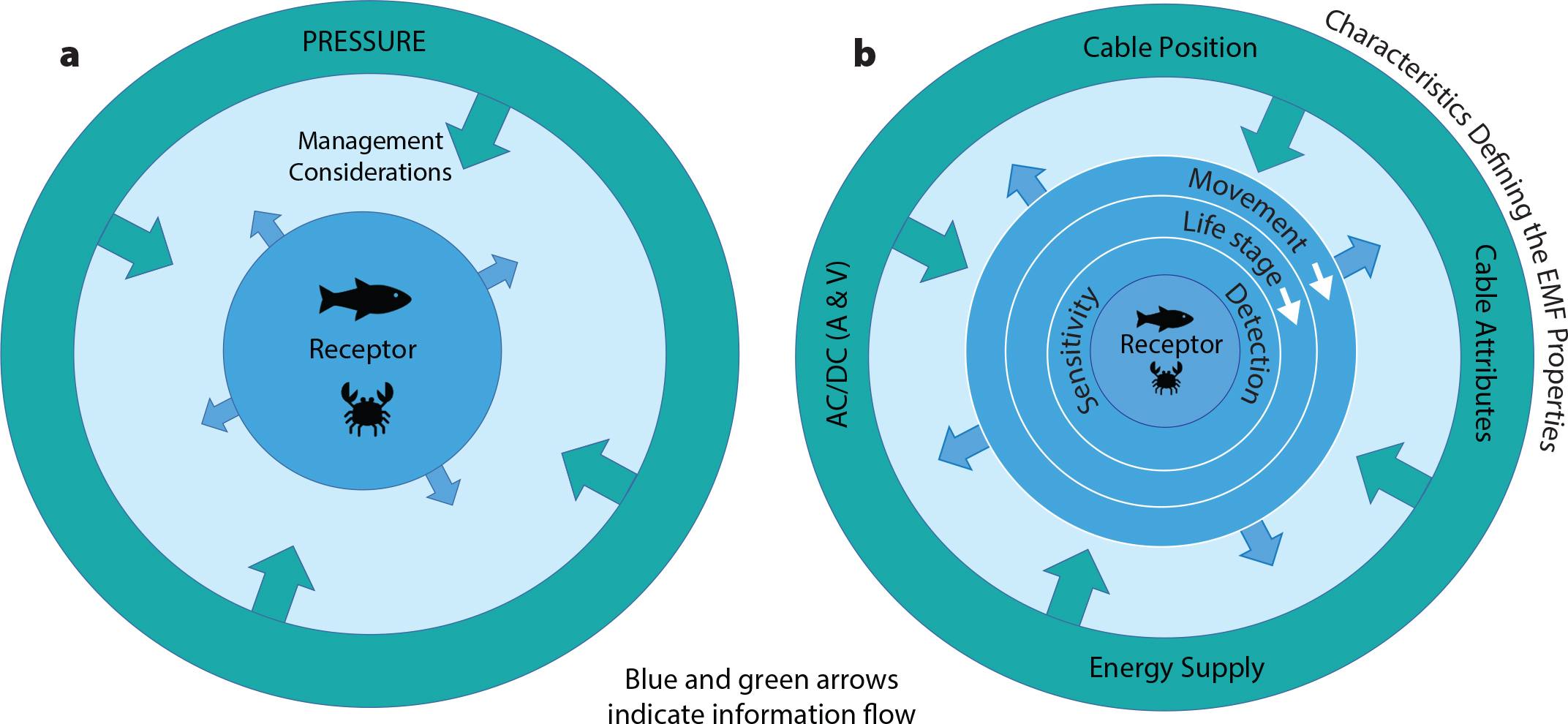
Figure 2. Vantage point of the receptor species. (a) Management must be informed by characteristics defining the pressure (here, EMF) and receptor response. (b) Sensory capabilities and detection thresholds are at the core of receptor species attributes and must be considered through the integration of life history ecology. Simultaneously, EMF characteristics must be known so that exposure levels can be determined and management can consider the likely encounter rate and potential consequences of exposure. A = Current (amps). V = Voltage (volts). > High res figure
|
Do You Sense That?
Specialized physiology allows receptive marine species to detect electromagnetic fields. Marine fish electroreception occurs through ampullary organs and/or lateral lines, both of which have existed throughout their evolution (Baker et al., 2013). Comparatively, marine magnetoreception is less well understood than electroreception in marine species, but the most likely mechanisms are magnetite-based and photochemical systems (Nordmann et al., 2017). Electroreceptors can detect the induced electric fields arising from movement of an animal or water body through a magnetic field, making electroreceptive organisms indirectly receptive to magnetic fields (Kalmijn, 1978). Some species may have both electro- and magnetoreceptive sensors (Anderson et al., 2017). The exact mechanisms of EMF detection are complex, but the best understanding to date is of the “ampullae of Lorenzini” (electroreceptors) in elasmobranchs (cartilaginous fishes of a group that comprises sharks, rays, and skates) (Tricas and Sisneros, 2004), making them good model species for advancing our understanding of EM-sensitive species.
EM-sensitive species may be receptive to anthropogenic EMFs that fall within the range of natural EMFs. The global geomagnetic field ranges from approximately 25 µT to 65 µT, and EM-receptive species are likely responsive to polarity, change in intensity, inclination and declination, and associated induced electric fields (Gill et al., 2014; Nordmann et al., 2017). These variations in natural EMFs are small scale and therefore suggest that marine species would be sensitive to very low level magnetic fields (nanotesla to microtesla; Walker et al., 2002); however, elasmobranchs are also responsive to much stronger magnetic fields (e.g., Porsmoguer et al., 2015). Generally, electrosensitive species are primarily responsive to both DC and AC low intensity electric fields between 0.02 μV cm−1 and 100 μV cm−1 and frequencies of 0–15 Hz (Tricas and Sisneros, 2004; Stoddard, 2010). Bedore and Kajiura (2013) measured bioelectric fields of marine prey, including fish and invertebrates, at the body surface in the range of 7–319 µV (approximate mean electric potentials) with frequencies of 0.12–10 Hz (though injured specimens may reach 500 Hz). The estimated detection distance of these prey fields by elasmobranchs was found to be <1 m in this study, although the bioelectric fields topic was highlighted as poorly understood. Sensitivity ranges for magnetic and electric field detection are better understood for some taxa (e.g., elasmobranchs) compared to others where information is lacking (e.g., teleost fish [the most diverse group of fishes], crustaceans). Detection thresholds of EMF components likely vary among species, precluding generalizations regarding effects on receptive species.
Lens of Life History Ecology
EMF perception, based on detection and sensory ability, likely varies through a species’ life history, and movement ecology affects likely exposure to EMF based on the likely encounter rate. An understanding of population-level effects of EMFs requires knowledge of these species’ characteristics. Marine fishes (elasmobranchs, teleosts) exhibit three life history types: (1) opportunistic species that reproduce early and die young (e.g., anchovies), (2) periodic species that mature and die late, producing lots of small offspring (e.g., cod, tunas), and (3) equilibrium species that reproduce late and die late, producing few large offspring (e.g., sharks, rays) (Winemiller and Rose, 1992; Secor, 2015). Commercially important invertebrates largely fall into the opportunistic and periodic categories. Both opportunistic and periodic species are more resilient to environmental disturbances, particularly those impacting early stages (egg, larvae, juvenile): they manage to offset the disturbances by high fecundity, early dispersal, and natural mortality rates (Gross et al., 2002). Thus, detection of EMF effects, particularly in assessment of population impacts, is more likely for adults of species maturing late and for juveniles of equilibrium species, stages when local impacts on individuals represent a larger fraction of the population. From this perspective, elasmobranchs, adult cod, and lobsters, for example, are better model organisms than anchovy adults and lobster larvae.
Movement ecology frames EMF exposure dynamics. Understanding individual path variations, typically on the scale of hours to months (Nathan, 2008; Secor, 2015), informs whether exposure to EMF is likely. Benthic species are presumed to have a greater likelihood than pelagic species of encountering buried cables while pelagic species are more likely to encounter dynamic cables. Here, movement ecology challenges the traditional dichotomy of benthic (or demersal) versus pelagic species—which has traditionally been important for generalizing EMF exposure. Vertical movements occur frequently in both demersal and pelagic species; demersal species (e.g., skates, cod, halibut) can regularly move in the water column (Nichol and Somerton, 2002; Hobson et al., 2007), and pelagic species (e.g., tunas, herring) sometimes depend on benthic habitats for foraging and reproduction (Chase, 2002; Overholtz and Friedland, 2002). Therefore, a pelagic species may still encounter the EMF of a buried cable if it is placed within an important benthic habitat. Likewise, a demersal species may encounter a dynamic cable. Thus, studies of exposure to cable EMFs must consider species movement ecology, which is often flexible and not exclusively demersal or pelagic. Movement ecology further emphasizes key habitat dependencies, including foraging, reproduction, predator evasion, and transit that are key considerations in sustainable fisheries (Fluharty, 2000).
THE PRESENT KNOWLEDGE BASE
A Brief Synthesis
The perception, biological relevance, and effects of EMFs vary throughout species’ lifetimes. For example, in benthic elasmobranchs (skates/rays), sensitivity and relevance changes throughout the life cycle, from predator detection in early development to communication and finding mates in adults (Sisneros et al., 1998). Similarly, in some species, young and/or adults use cues from the geomagnetic field during long distance migrations or in homing behaviors (Klimley, 1993; Putman et al., 2013; Cresci et al., 2017). A magnetic map sense and/or a magnetic compass sense facilitates navigation, allowing an animal to determine its position and direction in relation to a goal (Boles and Lohmann, 2003). Electroreceptive bentho-pelagic species also use navigational cues from geomagnetic fields (Kalmijn, 1978; Anderson et al., 2017); however, studies have predominantly focused on perceptions of prey-related electric fields. Moving from benthic to bentho-pelagic and migratory species, we explore these concepts in relation to effects of cable EMFs and independent electric or magnetic fields.
Focusing first on benthic invertebrates, the spiny lobster Panulirus argus provides the best example of true navigation, demonstrated in their annual migrations and homing behaviors (Lohmann et al., 1995). After geographical displacement and sensory limitation, lobsters were able to orient in the direction of their capture site, deriving their positions and directions from the geomagnetic field (Boles and Lohmann, 2003). It was thought that other crustaceans might share magnetoreceptive navigational senses, therefore lobsters and crabs became focal species for EMF studies. The American lobster (Homarus americanus) exhibited an exploratory response when exposed to a high voltage DC (HVDC) cable EMF compared to the local geomagnetic field (Hutchison et al., 2020). Later, in aquarium studies, juvenile European lobsters (H. gammarus) appeared to show no behavioral responses to magnetic field gradients (Taormina et al., 2020a). This discrepancy may be explained by species or life stage specific responses, or different exposure properties—aspects also relevant to contrasting crab studies. In situ choice chambers allowing crabs (Metacarcinus anthonyu, Cancer productus) to get closer to or farther away from an energized or unenergized cable revealed no preferences relating to EMFs (Love et al., 2015). Studies of the same species showed crabs crossing cable EMFs to enter baited traps (Love et al., 2017). However, aquarium studies showed that crabs did respond to magnetic fields. The Dungeness crab (M. magister) spent less time buried and, although the response was variable, exhibited more frequent changes in activity in the first two days of EMF exposure (Woodruff et al., 2012). In contrast, an attraction to exposed shelters was observed in the crab C. pagurus, coupled with reduced roaming and disrupted cycles of metabolic markers (Scott et al., 2018). Smaller crustaceans and mollusks have received less attention, but cellular responses have been recorded in some bivalves (Bochert and Zettler, 2004; Malagoli et al., 2004; Stankevičiūtė et al., 2019). Although benthic invertebrates have been given some attention, the methods, exposures, and results have been variable and, in some cases, contradictory.
Bentho-pelagic electroreceptive foraging species are perhaps the most well studied. Electroreception permits detection of cryptic prey and is the dominant elasmobranch sense when close to prey (Kalmijn, 1971). In the laboratory it has been shown that benthic predatory catsharks (Scyliorhinus canicula) can differentiate between types of electric fields and may be able to learn from experiences but only in the short term (Kimber et al., 2011, 2014). The observed preferences for higher DC EMFs suggest they may seek larger or injured prey, and preferences for AC over DC may reflect a choice of fish over invertebrates. However, no differentiation between natural and dipole DC fields occurred, suggesting that they may not distinguish between prey bioelectric fields and anthropogenic EMFs. Subsequent elasmobranch field studies support this hypothesis, showing foraging behavior in response to cable EMFs. A higher number of S. canicula were found in EMF zones of an AC cable and moved less, suggesting foraging behavior (Gill et al., 2009). In little skate (Leucoraja erinacea), a striking behavioral response was observed when exposed to an HVDC cable EMF compared to the control (Hutchison et al., 2020). The skates traveled much longer distances slowly, with more large turns, and they swam closer to the seabed. They also spent more time associated with areas of stronger EMF within the enclosure.
“Detection thresholds of EMF components likely vary among species, precluding generalizations regarding effects on receptive species.”
|
Turning our attention to prey, some initiate a “freeze response” to electric fields, mimicking predators. Prey within egg capsules (e.g., rays, sharks) briefly stopped ventilatory behaviors, including tail beating and gill movement, thus concealing their bioelectric field from predators (Sisneros et al., 1998; Kempster et al., 2013; Ball et al., 2016). There is, however, no knowledge as to whether predator detection may be masked by anthropogenic EMFs.
Formicki et al. (2019) documented other early stage effects of magnetic fields in some fish (gametes, sperm mobility, fertilization rate, embryonic development). Furthermore, Putman et al. (2014) show that the EM environment of early life stages may influence EM perception in later life stages. Cable EMFs associated with OSW may seem relevant only to species found offshore, but cable routes may pass through multiple ecosystems. Thus, cable EMFs are relevant to species in freshwater and estuarine environments as well, and these settings may harbor early life stages of species that spend later life stages offshore.
Migratory homing species navigate multiple ecosystems to get to important feeding or spawning grounds. Salmonids and anguillid eels are better studied than other such migrators. Directional changes in salmon fry, smolts, and adults (Onchorhynchus spp.) indicate a magnetic compass sense, and geomagnetic imprinting was revealed to facilitate Pacific salmon in navigating their way home to reproduce (Putman et al., 2013; Formicki et al., 2019). The magnetic compass in displaced adult eels was demonstrated, and in glass eels it was linked to the tidal cycle, suggesting endogenous regulation (Durif et al., 2013; Cresci et al., 2017). However, few studies assess interactions of migratory species with cable EMFs. Eels that encountered an AC cable on their outward migration slowed down but passed over the cable (Westerberg and Lagenfelt, 2008). In contrast, a study of salmon smolts swimming parallel to an HVDC cable moved faster, and while there appeared to be no barrier to movement, misdirection increased their journey to the sea (Wyman et al., 2018). Although cable EMFs potentially influence homing and associated reproduction in species such as Atlantic herring (Clupea harengus), which show natal homing to the same seabeds annually (Corten, 2002), they have not been studied.
Multi-species migration corridors or flyways may co-occur with OSW developments (Rothermel et al., 2020). Seasonal migrations of EM-receptive species (e.g., salmons, cod, tunas) may encounter multiple OSW cables. One expectation is increased stopovers by migratory fish that are attracted to OSW structures (foundations, scour/cable protection; the “reef effect”; see Degraer et al., 2020, in this issue). Increased dwell time (Gauldie and Sharp, 1996) may increase exposure to cable EMFs, with unknown orientation and navigational consequences. Furthermore, dynamic cabling (Figure 1) may present greater exposure to EMFs by pelagic species (sharks, tunas, cetaceans) and highly migratory species using flyways (Walli et al., 2009).
A Patchwork of Knowledge from a Variety of Methods
Field studies are advantageous in that they can assess cable EMF effects at true scales of influence and responses directly related to ecology. However, they can be disadvantaged by effect size (Type II error) (Franco et al., 2015) and confounding variables (e.g., reef effects, environmental forcing) (Taormina et al., 2018; Wyman et al., 2018). To overcome these issues, natural experiments and mesocosm trials have been adopted.
Natural experiments allow organisms to be observed interacting with cable EMFs. Examples include telemetry before/after studies of migrating species in systems with new cables (Wyman et al., 2018), studies of EMF-gradient influences on swimming speeds (Westerberg and Lagenfelt, 2008), and direct observations of fishes under varying cable EMF emissions (Kilfoyle et al., 2017). Effect size remains an issue but is diminished by targeted observing systems and careful experimental design. An important design component is selecting a sensitive model species (lobsters, elasmobranchs, sturgeons, cod) where key movement behaviors (small home range, homing, seasonal migrations) are well known (Boles and Lohmann, 2003; Dean et al., 2014). For instance, US Mid-Atlantic OSW development will expose important seasonally migrating (north–south, inshore–offshore) finfish and elasmobranchs to EMFs, as their movements will periodically cross cables (Rothermel et al., 2020). Careful baseline studies are key in evaluating EMFs at various scales of potential impact, including cumulative impacts on migration behaviors. Still, distinguishing cable EMF effects from structure attraction or nearby fishing activity will be impossible without additional controls on the experimental setting.
Mesocosm studies can provide additional control. They allow in situ exposure of test animals to control and EMF treatments and facilitate focusing on EMF variables and responses of interest while increasing sensitivity (reduced Type II error) through replication and other design elements. Coupled with fine-scale telemetry, mesocosms permit careful assessment of a wide array of movement behaviors and associated functions (Gill et al., 2009; Hutchison et al., 2020). Other study enhancements include testing homing or feeding behaviors by exposing the same test system to different conditions (cable, seabed) or performance of choice experiments (Love et al., 2017). The disadvantages of mesocosm experiments are that movements are necessarily constrained, mesocosm replication is expensive, and such studies are depth limited.
“Importantly, the context of effects must be related to the likely encounter rate, which must consider species ecology as well as cable properties. Accomplishing this is complex.”
|
In contrast to in situ studies of interactions with cable EMFs, laboratory studies have focused on independent magnetic or electric fields. An advantage of laboratory studies is that the type (AC/DC) and intensity of the field can be controlled. Simple electrical dipole fields can replicate prey-type electric fields that are similar in intensity and frequency to the electric field component of cable EMFs (Kimber et al., 2011). Likewise, static (DC) and time-varying (AC) magnetic fields can be produced by Helmholtz coils at intensities similar to those emitted by cables (Taormina et al., 2020a) and the geomagnetic field. A uniform field or gradient can be achieved in two or three dimensions by varying the number and configurations of coils (Kirschvink, 1992). Resulting complexities must be appropriately considered along with the ambient magnetic field to avoid becoming confounding factors. Although knowledge of species’ sensory abilities is patchy, an advantage of laboratory studies is that they can be used to decipher biochemical/physiological (cellular, genetic, developmental) and behavioral responses to individual field components (Scott et al., 2018; Formicki et al., 2019; Newton et al., 2019). However, laboratory results are sometimes ambiguous, leading to inferring/dismissing receptive abilities that may or may not be present. Furthermore, selection of suitable field intensities and endpoints is very important, but difficult to get right.
As highlighted by the present patchwork of knowledge available regarding sensory abilities and responses for numerous species at variable levels of exposure, there is much still to learn. Context-relevant studies that assess responses to anthropogenic EMFs are presently lacking (Newton et al., 2019; Nyqvist et al., 2020). A key future requirement is to demonstrate whether laboratory studies of individual component fields are representative in terms of their characteristics, geometries, and ranges of intensity in comparison to cable EMFs and their interactions with ambient electromagnetic environments. Such an evaluation will allow better analysis and interpretation of the context in which receptive organisms encounter EMFs.
EMF Effect Size Amidst Shifting Baselines
In temperate marine shelf ecosystems, natural and anthropogenic disturbances and altered environments (Mapstone, 1995) that are due, for example, to climate change and storms may overshadow effect sizes. Storms are key natural disturbances that can rapidly alter conditions and influence fish behavior in OSW areas (Secor et al., 2019). Thus, to evaluate EMF impacts on receptive species requires baseline studies that account for the effects of storms and climate change. Furthermore, OSW structures (cable/scour protection, foundations) may elicit responses and influence the likely EMF encounter rate. Understanding the relative EMF effect size requires careful field experimentation to guide management (Mapstone, 1995; Wilding et al., 2017).
MARINE MANAGEMENT AND MOVING FORWARD
Environmental impact assessments identify problems that are likely to affect resource species and ways to mitigate any effects. These assessments are reviewed in light of available knowledge of both the pressure and the receptor. While the knowledge base of how species interact with EMFs has grown, the evidence base informing assessments and management is still lacking. Furthermore, unlike on land, there are no relevant regulations or policies concerning EMFs for marine environments (e.g., human health; WHO, 2020). Indirectly, however, legislative frameworks or regulations do prevent energy emissions (including EMFs) from adversely affecting marine biodiversity status (EU Marine Strategy Framework Directive) or designated species of conservation (EU Habitats Directive).
Making informed decisions requires an improved knowledge base for both the pressure and receptor. Figures 3 and 4 highlight the desired knowledge bases and steps needed to reach them. Focusing first on the pressure (Figure 3), a better understanding of the factors that influence EMFs is needed, including cable properties and power transmission variations. Measuring cable EMFs at scales relevant to receptive species requires the development of affordable, validated methods. Characterization of the cable EMF should also consider the local environmental characteristics, namely the local geomagnetic field, its geometry, as well as the interactions with anthropogenic EMFs (i.e., cables and other sources). Such measurements should inform appropriate modeling of future OSW scenarios. Standards for appropriate measurement and reporting of EMF environments, as relevant to receptive species, would be beneficial, and encouraging and facilitating data sharing would assist in more readily advancing understanding.
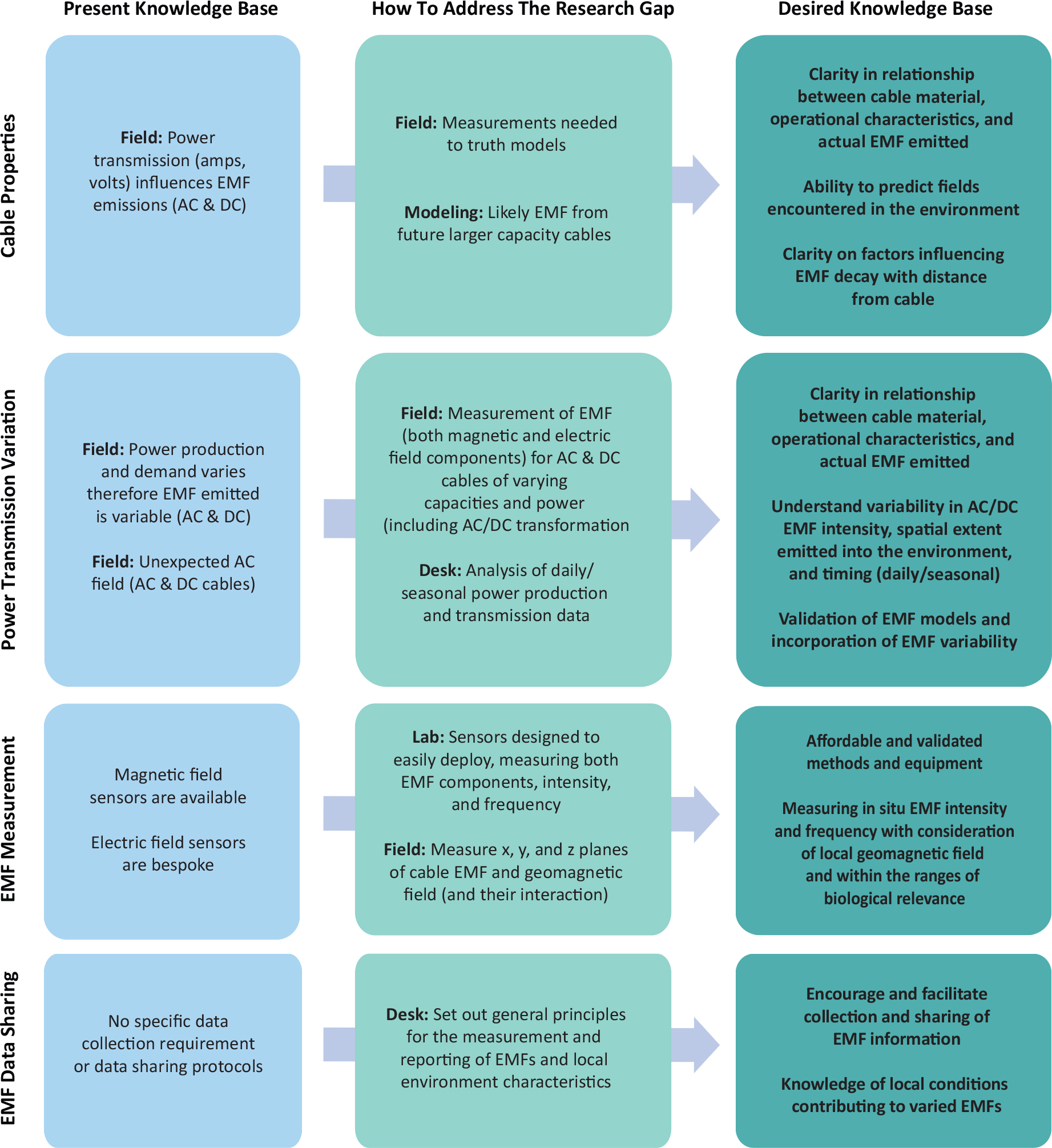
Figure 3. Pressure related present and desired knowledge base. The present knowledge base is summarized (left) bridged to the desired knowledge base (right) by methods addressing knowledge gaps (center). > High res figure
|

Figure 4. Receptor related present and desired knowledge base. The present knowledge base is summarized (left) and is bridged to the desired knowledge base (right) by methods addressing knowledge gaps (center). Sensory, life history, and movement ecology are integrated. Field and laboratory methods require careful consideration of species and experimental endpoints. > High res figure
|
For receptor species, it is difficult to translate the patchwork of knowledge about individual-level EMF effects into assessments of biologically or ecologically significant impacts on populations (Boehlert and Gill, 2010). Presently, improving the “effects” knowledge base using model species would be most beneficial. In some cases, specific resource species may need to be considered. Overall, efforts to improve the effects knowledge base should be aimed at understanding population-level impacts, incorporating aspects of life history and movement ecology (Figure 4). Importantly, the context of effects must be related to the likely encounter rate, which must consider species ecology as well as cable properties. Accomplishing this is complex. Presently, because more OSW developments use more AC cables than DC cables (Soares-Ramos et al., 2020), marine animals are more likely to encounter AC cables. Models show that DC cables emit EMFs at higher intensity and over a greater spatial extent than AC cables (Gill et al., 2014), suggesting a lower likelihood of EMF encounter for AC cables (i.e., lower spatial reach, lower intensity). However, this interpretation presumes that higher intensity EMFs elicit greater effects (e.g., avoidance behavior), but low-intensity EMF may be more biologically relevant (e.g., mimic prey bioelectric fields with no food to be gained) and may be more frequently encountered, which could be deemed more of an issue.
Finally, cumulative effects are both physical and biological. Physically, more numerous cables, their orientation, and cable type may influence EMFs encountered by marine fauna. Biologically, behavioral and physiological effects may interact, early life history experiences may influence later life stages, and a single encounter may inform the next exposure, or not. Further, EMFs may need to be considered along with OSW-associated infrastructure risks such as entanglement or reef effects (Degraer et al., 2020, in this issue; Taormina et al., 2018, 2020b).
Marine managers clearly have a challenging task concerning the assessment of potential impacts of EMF on marine animals due to the lack of knowledge. Taking the vantage point of the receptive species highlights that it is both their perception of EMFs and their exposure to them that is relevant in assessments of effects and potential impacts. Knowledge of the characteristics of both the EMF environment and receptive species must be integrated into these assessments (Figure 2). With future plans for more expansive OSW arrays that are located at greater distances offshore and use larger capacity power cables, a higher encounter rate is certain. While obtaining the desired knowledge base will be challenging, it will either help reduce the risk of EMFs to important resource species or retire the risk with more confidence.
ACKNOWLEDGMENTS
We thank Carol Gibson (University of Rhode Island) for assistance with figure preparation and the guest editors for the invited contribution on this topic. We also thank the reviewers for their valuable inputs.






