Introduction
With a global cumulative capacity of 651 GW installed, wind is one of the most exploited sources (GWEC, 2019) in the world’s transition toward renewable energy. Given the need for space, wind energy developments are typically constructed in vast open landscapes, which are scarce in most European countries and in highly populated coastal areas elsewhere but remain largely available at sea. Offshore wind farms (OWFs) currently represent only 4.5% of installed wind capacity. In 2019, there was a global addition of 6.1 GW, and the yearly addition is projected to double by 2024 (GWEC, 2019). OWFs are proliferating in Europe, mainly in the North Sea, but have also gained momentum in China and are advancing along the US East Coast with more than 15 OWF projects projected to be built by 2026 (https://www.4coffshore.com/offshorewind/).
While OWFs are typically less frequently confronted with a NIMBY (“not in my back yard”) attitude than those on land, they are, however, approached with reluctance by many ocean users. Since the construction of the first OWFs, concerns have been raised about economic costs and benefits, as well as their effects on natural environments (Devine-Wright and Wiersma, 2020). Such concerns continue to be strongly raised by the commercial fishing community, spawning such newspaper headlines as: “Wind farms taking grounds and damaging marine life” (Fishing News, 2019) and “Fish are kept away by wind turbines” (translated from “Vissen blijven weg door windmolens”; De Krant van West-Vlaanderen, 2018). On the other hand, recreational fisheries tend to see OWFs as a blessing because they provide excellent angling opportunities, with increased abundances of their favorite fish, for example, at Block Island Wind Farm (Rhode Island, USA; ten Brink and Dalton, 2018).
OWFs do change the local environment above and below the sea surface (Lindeboom et al., 2015). The most obvious and well-studied negative impacts above the sea surface have been detected for species of conservation value. Several seabird species such as guillemots (Uria aalge) and northern gannets (Morus bassanus) show a distinct avoidance of operational OWFs (Skov et al., 2018). Other seabirds such as larger gulls seem attracted to the OWFs and run the risk of colliding with the turbine blades (Vanermen et al., 2020). Below the sea surface, marine mammals such as the harbor porpoise (Phocoena phocoena) flee the area during pile-driving activities (Brandt et al., 2018), and highly migratory fish such as tuna (Thunnus spp.) may be disturbed by the operational sounds of OWFs (Espinosa et al., 2014).
OWFs may also have more obscure effects on marine wildlife that could be perceived positively. Many top predators seem to target the OWFs for food and/or refuge and profit from the ecological changes that take place following their installations (see below). Additionally, OWFs are known to affect the benthos and demersal and bentho-pelagic fish (Dannheim et al., 2020). These changes, mainly below the sea surface, are commonly referred to as the “artificial reef effect.”
Artificial reefs are man-made structures (i.e., hard substrates) deliberately placed in the sea to mimic characteristics of natural reefs. The term “artificial reef” has been in the literature since the 1930s (Bohnsack and Sutherland, 1985), but structures aimed at promoting fisheries and aquaculture have been around for at least 5,000 years (Tickell et al., 2019). The most common purpose for deploying artificial reefs has been to improve biodiversity, particularly with respect to fishery species (Bohnsack and Sutherland, 1985). However, structures that function as artificial reefs are not always purpose built. Today, such structures have become a side effect of “ocean sprawl,” a term that reflects the proliferation of man-made structures in the sea such as oil and gas platforms, aquaculture cages, coastal defense constructions, and OWFs (Firth et al., 2016).
This article provides an overview of the artificial reef effects of OWFs on ecosystem structure and functioning. We focus on how OWFs provide new habitat, setting the stage for colonization by epifaunal communities consisting of species that are both indigenous and nonindigenous, of conservation interest, and that have habitat-forming properties. We also consider local organic enrichment, subsequent influences on the benthos of the surrounding sediments, and the attraction of predators and scavengers. Finally, we provide some insights into the spatial extent of OWF artificial reef effects and how best to deal with them. We particularly aim to provide lessons learned from European and American studies in the North Atlantic. This article does not examine how the presence of OWFs may exclude fisheries, which is considered to be only a secondary component of the artificial reef effect, and which is covered by Gill et al. (2020) in this special issue of Oceanography.
It all starts with biofouling of a newly introduced habitat…
It is now widely accepted that one of the most important effects of OWFs is the provision of new habitat that can be colonized by hard substrate species (Petersen and Malm, 2006). Setting aside the loss of soft sediment habitat due to the OWF footprint, OWF structures generally provide two distinct artificial habitats: hard vertical substrates and a complex range of horizontal habitats, depending on the type of foundation and the degree of scour protection used (Langhamer, 2012). In addition, the novel surfaces occur throughout the full water column, from the splash zone to the seafloor, often in areas where comparable natural hard surfaces are absent. These attributes are largely unique to offshore energy infrastructure. The introduction of coarse rock affects seabed habitat complexity, particularly in mobile sediments, expanding the habitats available to serve as refuges and to support food sources for biota. In Europe, most OWFs are constructed in mobile sedimentary environments, but in the northeastern United States, several OWFs are proposed for installation on glacial moraines that have high densities of boulders mixed with mobile sediments (Guarinello and Carey, 2020).
Biofouling Community Structure and Succession
Installation of any new OWF has invariably been followed by rapid colonization of all submerged parts by a variety of fouling organisms that are familiar from studies of other anthropogenic structures placed in the marine environment (e.g., Kingsbury, 1981; Schröder et al., 2006). Vertical zonation is observed on the turbine foundations, with different species colonizing the splash, intertidal, shallow, and deeper subtidal zones (De Mesel et al., 2015; Figure 1). In general, biofouling communities on offshore installations are dominated by mussels, macroalgae, and barnacles near the water surface; filter-feeding arthropods at intermediate depths; and anemones in deeper locations (De Mesel et al., 2015). In the southern North Sea, adult mussels are rare at deep offshore locations that do not have hard substrate near the water surface. However, OWF structures provide a novel mussel offshore habitat, with high abundances exhibited on turbine foundations (Krone et al., 2013a). Larger species such as crabs and lobsters appear to profit from the presence of the structures and the biofouling community, appearing in increasing abundance on and around the structures (Krone et al., 2017). At the scale of a turbine footprint, biomass can increase 4,000-fold compared to the biomass originally present in the sediments (Rumes et al., 2013). OWF structures may also affect communities living on surrounding natural hard substrates such as boulder fields. The biota attracted by their dominant vertical surfaces, high depth ranges, and different surface textures and compositions might affect the assemblages of invertebrates and algae resident on nearby boulders (Wilhelmsson and Malm, 2008).
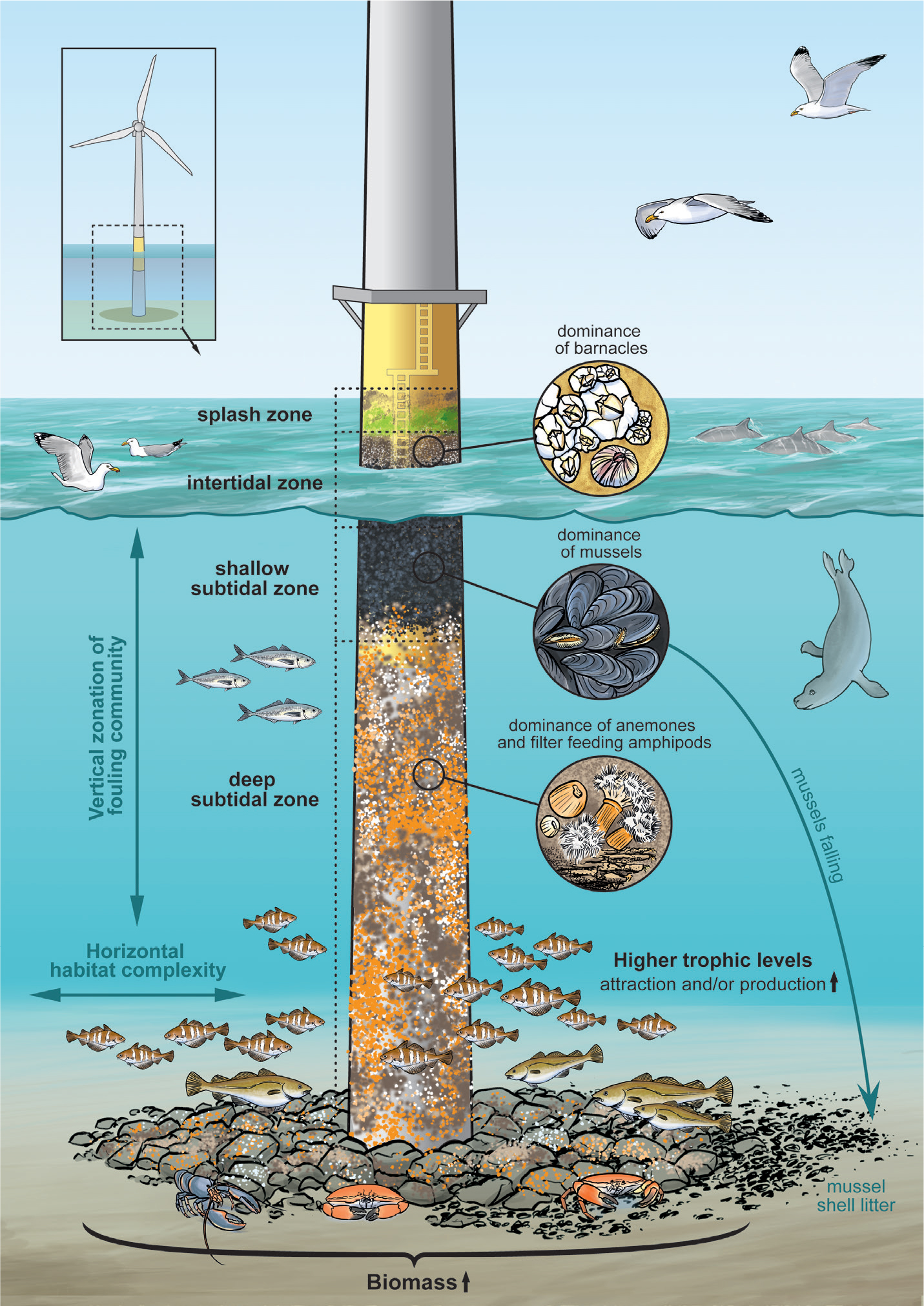
Figure 1. Offshore wind farm structures provide habitat for invertebrate organisms that foul the foundation along the depth gradient and attract predator fish, seabirds, and marine mammals. Illustration by Hendrik Gheerardyn. > High res figure
|
There are currently five types of offshore wind turbine structures in use: monopiles, gravity-based foundations, jacket and tripod structures, and, more recently, floating wind structures. Each structure has obvious differences in submerged surface area and structural complexity (Rumes et al., 2013). The exact influence of the structure type on the degree of the artificial reef effect has not yet been quantified.
Over time, the initial set of species can evolve into a highly biodiverse community composed of many species from a large number of phyla (Coolen et al., 2020a). Much of the information documenting the colonization and succession process on OWF artificial hard substrates is derived from short-term time series or one-off sampling events. These studies focused on the high species richness on the structure compared to surrounding soft sediments. The only long-term (10-year) study identified three distinct succession stages (Figure 2): a relatively short pioneer stage (0–2 years) was followed by a more diverse, intermediate stage (3–5 years) characterized by large numbers of several suspension feeding invertebrates, and a third “climax” stage (6+ years) co-dominated by plumose anemones (Metridium senile) and blue mussels (Mytilus edulis) (Kerckhof et al., 2019). This climax stage is in line with observations at offshore oil and gas platforms where mussels mixed with hydrozoans and anemones dominated the older and deeper sections (~15–50 m) (Coolen et al., 2020a). In general, the vertical section of offshore foundations forms a uniform habitat that, in the long term, allows a few competitive species to dominate the fouling communities.
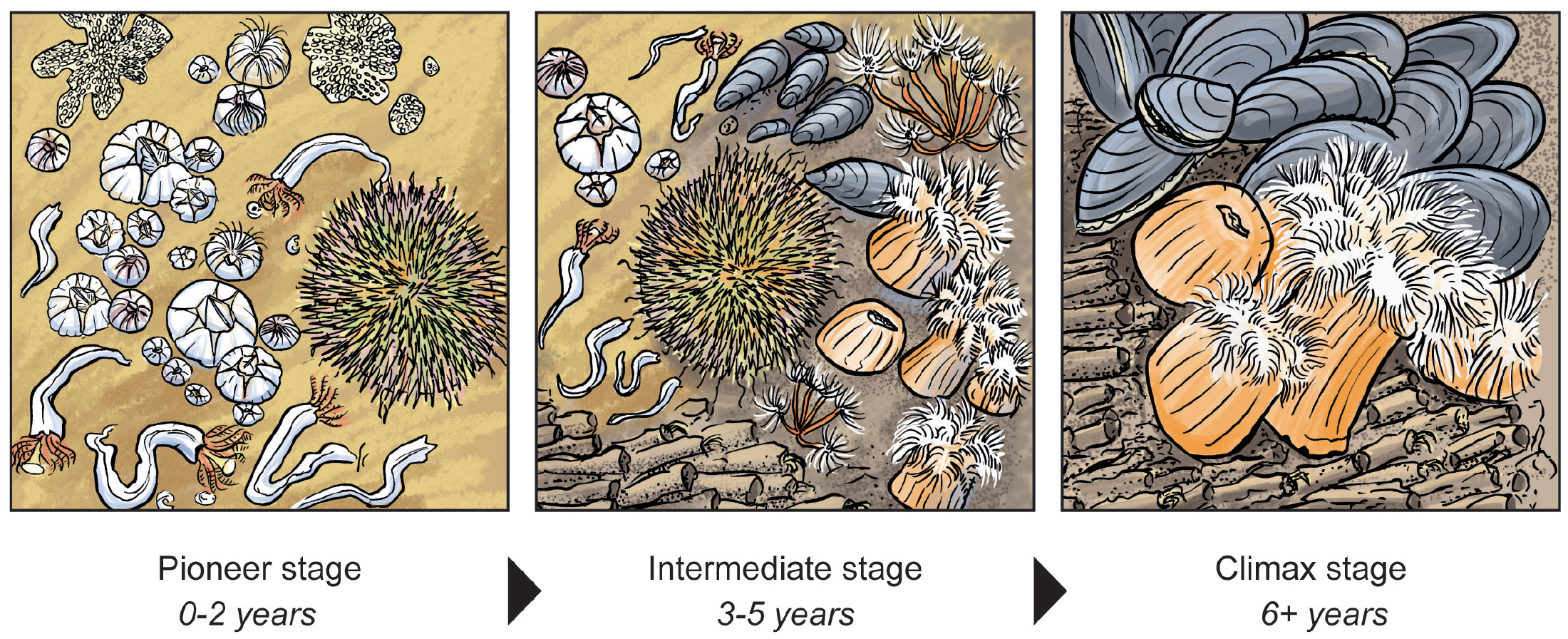
Figure 2. The colonization of offshore wind turbines passes through clear successional stages: a pioneer stage with a few early colonizers, a species-rich intermediate stage, and a climax stage dominated by mussels and anemones. Illustration by Hendrik Gheerardyn. > High res figure
|
OWF scour protection, which typically consists of rocks of varying sizes and shapes intermittently covered by sand, provides additional microhabitats for a multitude of species. While physically it more closely resembles natural rocky reef habitats, its fauna remains distinctly different from those found among natural hard substrates (Coolen et al., 2020a). Research in Belgian and Dutch waters is targeting the feasibility of fine tuning the design of scour protection to contribute to the restoration of the natural gravel bed ecosystems lost about a century ago.
Nonindigenous, Rare, and Habitat-Forming Species
Ocean sprawl in shallow and coastal waters provides opportunities for nonindigenous species. In the shallow southern North Sea where OWFs were first installed, nonindigenous species were indeed found among the colonizing species, for example, the Pacific oyster (Crassostrea gigas) and the marine splash midge (Telmatogeton japonicus) (De Mesel et al., 2015). The highest number of nonindigenous species were found in the intertidal and splash zones. These habitats are largely new to the open sea and offer an empty niche for nonindigenous species to extend their distributions and/or strengthen their populations. Subtidally, records of nonindigenous species are more scarce. For Belgian OWFs, only one nonindigenous species (the slipper limpet Crepidula fornicata) was recorded in subtidal samples. In the Netherlands, however, six out of eleven nonindigenous species were found subtidally (Coolen et al., 2020a). At Block Island Wind Farm, the widespread nonindigenous and proliferating ascidian Didemnum vexillum was observed on both the foundation structure and as an epibiont to the mussels (HDR, 2020). As yet, there are no published records of range expansion of subtidal nonindigenous species relating to the introduction of OWFs. While there is concern that OWFs may pose a threat to indigenous communities (Glasby et al., 2007; Adams et al., 2014), this threat has yet to be demonstrated.
The drastic increase of hard substrates in an environment consisting largely of soft mobile substrates can favor the spread of hard substrate species by creating new dispersal pathways and facilitating species migrations, the so-called “stepping stone effect” (Adams et al., 2014). In the North Sea, southern hard substrate species such as the barnacle Balanus perforatus have now expanded further north, making use of the (intertidal) habitat provided by the OWFs (Glasby et al., 2007; De Mesel et al., 2015).
Several locally rare species, some of which are of conservation interest either because of their threatened status or because of the habitat they create, have taken advantage of the new habitat provided by OWFs. It is important to understand the role of this artificial habitat in maintaining local populations of these species, as it is likely to have implications for future decommissioning of OWFs (Fowler et al., 2020). At several OWFs, for example, fish species that prefer hard substrate, and therefore are either unknown or extremely rare on the surrounding sandy seabed, have been recorded in association with the structures’ artificial hard substrates (Van Hal et al., 2017). As the size, number, and geographic distribution of artificial reef habitat increases with the expansion of OWFs, additional fish species with affinities to hard substrates are likely to occupy this habitat. OWFs may therefore contribute to these fish species’ population sizes, extents, and connectivity. Furthermore, by providing small patches of appropriate habitat in otherwise unsuitable surroundings, artificial reefs can sustain local populations and even affect the spatial distribution of sessile hard substrate species formerly unknown to the area (Henry et al., 2018). An example is the appearance of the northern cup coral (Astrangia poculata) at the Block Island Wind Farm (HDR, 2020), and in the North Sea, such species include the stony coral (Desmophyllum pertusum) and the European flat oyster (Ostrea edulis) (Henry et al., 2018; Kerckhof et al., 2018). Given enough time, these reef-forming species associated with hard substrate may develop secondary biogenic reefs that could provide a home to many—often rare—species and offer great value with regard to ecosystem functioning (Fowler et al., 2020).
The most predominant colonizing species on OWFs, the blue mussel (Mytilus edulis) may have profound bioengineering and reef-building effects on the surrounding sediments. For example, mussel shell litter and layers of mussels falling from turbines may provide habitat for other species (Krone et al., 2013b), and “drop-offs” may be transported, introducing them to areas further from the turbines (Lefaible et al., 2019). Furthermore, evidence of adult blue mussel aggregations with distinct macrofaunal communities has been found on soft sediment near turbines (<50 m) in Belgian (Lefaible et al., 2019) and US (HDR, 2020) waters. Continued monitoring is required to determine the spatial extent and longevity of these aggregations to determine their potential designation as reefs and whether these aggregations could contribute to restoring functions of bivalve reefs that historically consisted of Ostrea edulis beds in the North Sea (Bennema et al., 2020).
Altered Food Webs and Ecosystem Processes
Suspension Feeders and Local Organic Enrichment
Wind turbines are generally colonized by high densities of suspension feeders (Krone et al., 2013a; HDR, 2020). A large portion of the biofouling community feeds on food particles suspended in the water column that include phytoplankton, zooplankton, and detritus (Figure 3). The predominant blue mussel Mytilus edulis, for example, actively filters water and ingests particles from it. Other suspension feeders, such as amphipods like Jassa herdmani, grab particles from the passing water to eat and to build their tubes (https://www.marlin.ac.uk/). The highly abundant plumose anemone (Metridium senile) is a passive suspension feeder that extends its tentacles in the water, waits for particles to stick to them, and then takes the particles in. Over 95% of the biomass on artificial structures can be composed of various species of suspension feeders (Coolen et al., 2020b), several of which are highly resource flexible, switching between suspended food sources, possibly due to interspecific competition or benefiting from food sources available in abundance (Mavraki et al., 2020a). By filtering the water, the organisms remove particles that would have otherwise passed by, resulting in lower turbidity and increased light penetration. This “biofilter” effect has been demonstrated at the local scale (Reichart et al., 2017) and in the laboratory (Mavraki, 2020b) but may result in larger-scale effects when considering multiple offshore installations. However, in-depth understanding of this effect is currently lacking (Dannheim et al., 2020).
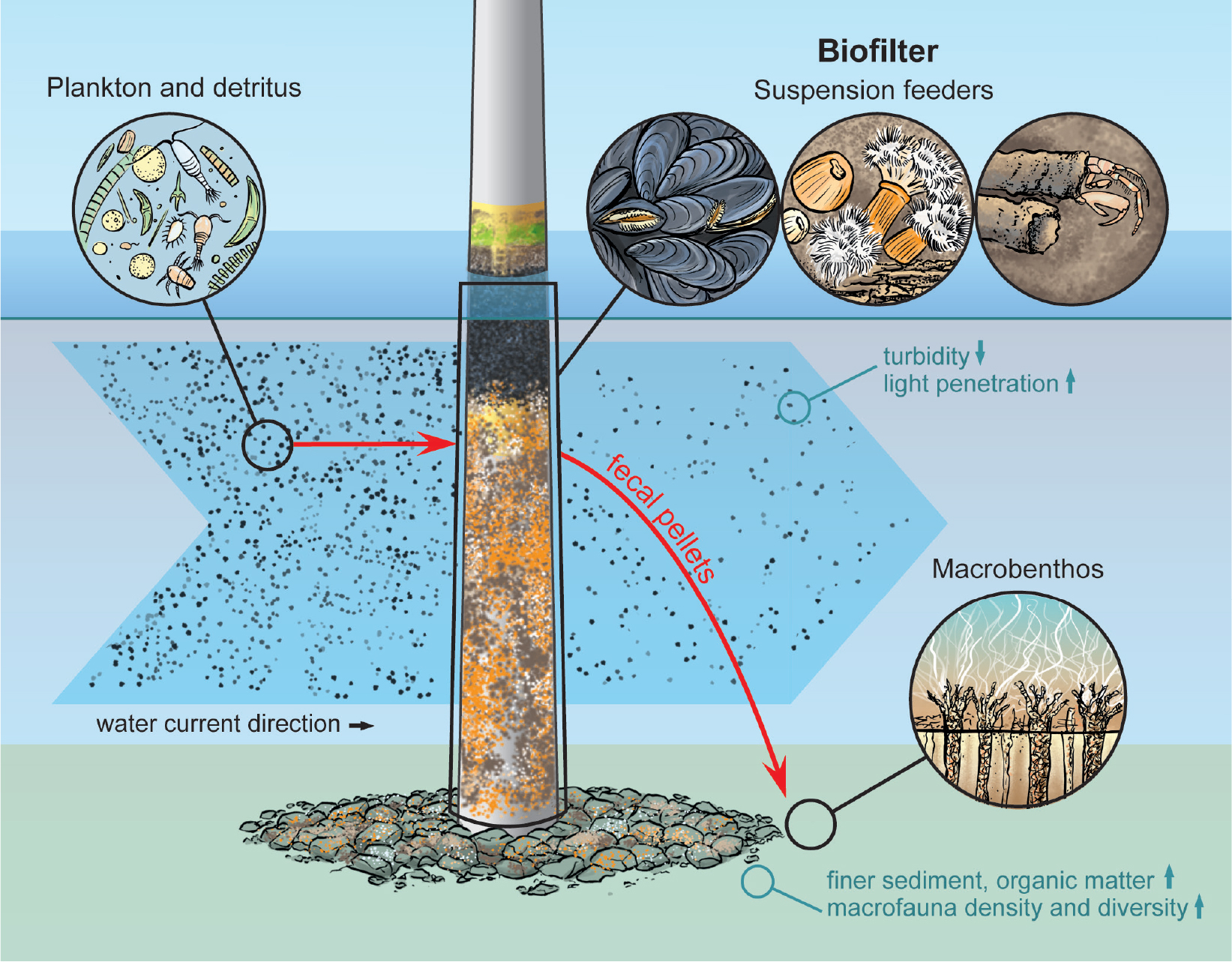
Figure 3. Offshore wind farm artificial reefs act as “biofilters,” hosting suspension feeders that filter organic matter from the water column and organically enrich the surrounding seabed. Illustration by Hendrik Gheerardyn. > High res figure
|
Model results suggest that the soft sediment around turbines can be enriched through the deposition of fecal pellets egested by these filter feeders (Maar et al., 2009). By consuming primary producers, the suspension feeders that constitute the biofilter make pelagic food sources available to the benthic community (Slavik et al., 2019), likely increasing secondary production in OWF artificial reefs (Krone et al., 2017; Roa-Ureta et al., 2019). This hypothesis was first tested around a single gravity-based foundation in the Belgian part of the North Sea (Coates et al., 2014). Later research targeted multiple jacket and monopile foundations in several Belgian wind farms (Lefaible et al., 2019) and the jacket foundations of the Block Island Wind Farm (HDR, 2020). Samples obtained from the seafloor close to the foundations (<50 m) three to six years after installation showed evidence of finer sediments and increased organic matter, but this was less evident for monopile foundations. At all turbine types, macrofaunal communities closer to the turbine showed increased densities and species richness and/or diversity when compared to communities sampled further away (Coates et al., 2014; Lefaible et al., 2019; HDR, 2020). At Belgian wind farms located in high-energy sandbank environments, soft sediment communities sampled closer to the jacket and monopile foundations displayed similarities with communities associated with lower-energy environments (Lefaible et al., 2019). As these findings are now reported for different turbine types and from different areas around the world, it is reasonable to consider changes in the sedimentary environment and associated macrofauna to be a typical feature associated with the installation of offshore wind farms. Whether these changes are solely linked to the localized deposition of organic matter by fouling fauna, and how these changes cascade into benthic ecosystem functioning, is still not clear and merits further investigation.
Higher Trophic Levels Profit from Increased Availability of Food and Shelter
Higher-trophic-level species with mobility appear to be attracted to the OWF structures for shelter and food availability. Studies of finfish distributions before and after installation of OWFs demonstrate that some finfish species, for example, Atlantic cod (Gadus morhua), pouting (Trisopterus luscus), black sea bass (Centropristis striata), and goldsinny wrasse (Ctenolabrus rupestris), spend at least part of their life cycles closely associated with the structures (Bergström et al., 2013; Reubens et al., 2014; Wilber et al., 2020). Increases in fish abundance around wind turbines, including flatfish such as plaice (Pleuronectes platessa) (unpublished data, Research Institute for Agriculture, Fisheries and Food, Belgium) may be caused by an attraction of individuals that aggregate near the new hard structures, with no net increase in the local population. Alternatively, production may be increased by the addition of new habitat that may enhance settlement, survival, and/or growth, or may save energy (Schwartzbach et al., 2020). The attraction/production mechanisms are not mutually exclusive (Brickhill et al., 2005). The original attraction/production hypothesis is complemented by a third option‚ the ecological trap, which refers to fish being attracted to suboptimal habitat, possibly leading to deterioration of the fish stock’s condition (Reubens et al., 2014).
Three types of species attracted to OWFs can be discerned: (1) species that predate the biofouling community for a prolonged period such as the Atlantic cod (Gadus morhua), the pouting Trisopterus luscus), and the Arctic sculpin (Myoxocephalus scorpioides) (Type A); (2) species that occasionally predate the biofouling community such as the Atlantic horse mackerel (Trachurus trachurus) (Type B); and (3) species such as the Atlantic mackerel (Scomber scombrus) that are attracted for nontrophic reasons, for example, to find shelter or to encounter other individuals of their species, which may lead to their creating larger schools and thus increasing their safety and chances of finding food and mates (Type C) (Mavraki, 2020). While a distinction may be made between benthic/bentho-pelagic (Type A) and pelagic (Type B or C) species, this distinction is not always clear. Aside from fish, other species attracted to OWFs by increased subtidal food availability include herring gulls (Larus argentatus) that forage in the intertidal zone of jacket-founded windmills (Vanermen et al., 2017) and common seals (Phoca vituline), with some individuals shown to make targeted foraging trips to Scottish OWFs (Russell et al., 2014).
Sphere of Influence
Numerous biotic and abiotic components within ecosystems exhibit multiple cause-effect pathways that operate over different spatial and temporal scales (Dannheim et al., 2020). With respect to the artificial reef effect, changes are most obvious at the scale of the turbine and its surrounding area. These first-order effects could be considered trivial in the context of the ecosystem, but small-scale changes are the basis of large-scale changes and can be used to inform potential regional impacts on components important to ecosystem services, such as commercial fish stocks (Wilding et al., 2017). The artificial reef effect, as detailed in this paper, is clearly not restricted to the structures themselves but rather extends in four dimensions (Degraer et al., 2018; Figure 4). This is evident not only in the changes in connectivity of benthic species facilitated by larval transport but also in the mobile fauna that make use of the whole wind farm, including those that do so seasonally and/or opportunistically (Reubens et al., 2014; Russell et al., 2014).
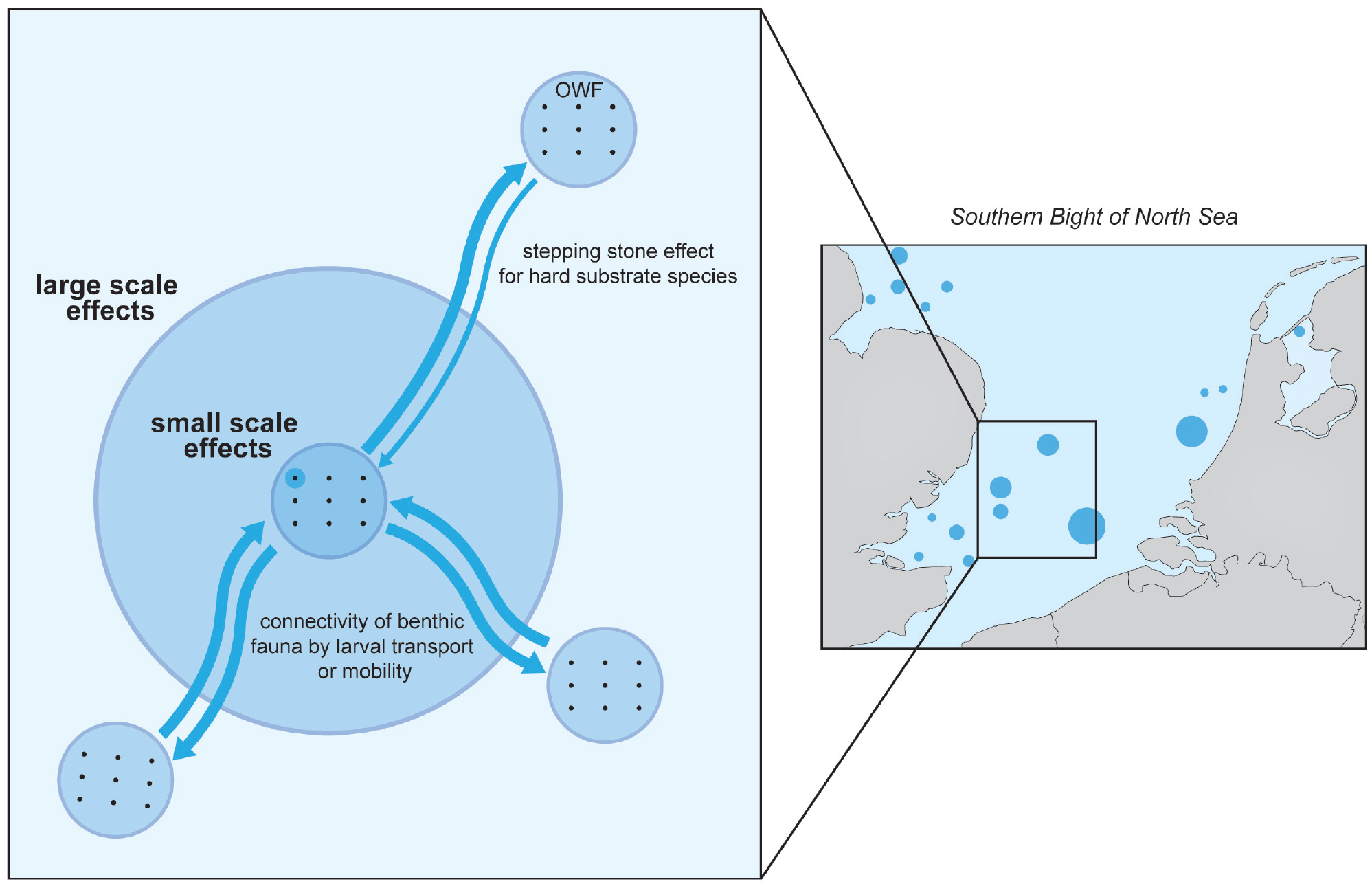
Figure 4. While the offshore wind farm artificial reef effect is particularly detectable at the scale of the wind turbine and the wind farm (small-scale effects), some effects extend well beyond the scale of a single such operation (large-scale effects) as exemplified by the increased connectivity of hard substrate species (the stepping stone effect). Illustration by Hendrik Gheerardyn. > High res figure
|
It is therefore important to account for the functional spatial and temporal scales of ecosystems or their parts in order to assess the artificial reef effect. Many adult fish, for example, show migratory behavior between spawning and feeding grounds that may extend several hundreds to thousands of kilometers. As planktonic organisms, many fish and invertebrate larvae move from spawning to nursery grounds over distances up to tens of kilometers (Lacroix et al., 2018). Hence, species may encounter OWFs for only limited parts of their lives and/or during very specific periods of their life cycles. In Belgium, for example, pouting (Trisopterus luscus) is known to feed on the invertebrate fouling organisms that colonize OWF structures and to do slightly better inside OWFs compared to outside (Reubens et al., 2014). This species is known to be attracted to OWFs only during the feeding and growing season in summer and autumn, after which they migrate to their spawning grounds outside Belgian waters (Reubens et al., 2014). Barbut et al. (2020) further showed a differential overlap between the spatial distribution of the spawning grounds of six southern North Sea flat fish species and the distribution of OWFs, assuming a species-specific effect of OWFs on the larval influx to the nursery grounds along the southern North Sea coasts. How these differential spatial and temporal effects translate to the population dynamics of those species affected by OWFs is key to our understanding of how the OWF artificial reefs impact marine ecosystems, but this is yet to be fully understood.
Where to go from here?
Priority Known Unknowns
The presence of the OWF structures and their concentrations of marine organisms have consequences for ecosystem functioning, at least at the local scale (Dannheim et al., 2020). Modeling efforts and experiments suggest local depletion of organic matter from the water column due to the activity of suspension feeders (Slavik et al., 2019). Suspension feeders transform the living pelagic organic matter pool into partially dissolved and bioavailable nutrients (Slavik et al., 2019) and produce (pseudo)feces that are partly deposited on the seafloor, as indicated by the increase in organic matter content around different types of turbines (Coates et al., 2014; Lefaible et al., 2019). While studies of the effects of aquaculture of the blue mussel (Mytilus edulis) yielded data on particle removal (Cranford, 2019) and its effect on pelagic and benthic nutrient cycles (Petersen et al., 2019), similar data for the other dominant fouling species in OWF environments are lacking. Availability of such data would allow estimation of the biogeochemical footprint of an OWF at the local scale. Further integration of such data in oceanographic models could allow assessment of the changes associated with multiple OWFs at a wider geographical scale.
Another “known unknown” is how artificial reefs affect carbon flow through locally altered food webs. Observations and modeling reveal increased abundance of fish (Reubens et al., 2014) and large crustaceans (Krone et al., 2017) as well as increased importance of a detritus-based food web. However, quantification of the carbon flow through the OWF food web is lacking. Such a study would require embracing well-established techniques such as stable isotope and fatty acid analyses, pulse-chase experiments, and food-web modeling approaches.
Finally, artificial reefs, like natural reefs, are being subjected to a warmer and acidified marine environment. The combination of acidification and warming leads to substantial, non-additive and complex changes in community dynamics (Queirós et al., 2015), affects pelagic and benthic nutrient cycling (Braeckman et al., 2014), and alters the mechanism behind predator-prey interactions (Draper and Weissburg, 2019). Thus, current understanding of the artificial reef effect in OWFs must be considered within a modern changing environment.
“The structural and functional effects of offshore wind farms extend in space and time, impacting species differently throughout their life cycles. Effects must be assessed at those larger spatiotemporal scales.”
|
Mitigating Undesired and Promoting Desired Effects
Although artificial reefs are often deliberately deployed to promote biodiversity, their net environmental benefits are often debated. For example, how should the eventual increase in fish productivity be balanced against the loss of fishing grounds? Although not designed as artificial reefs, OWFs have similar desired and undesired impacts: they may offer possibilities for nature enhancement, but at the same time be a nuisance to nature (Lindeboom et al., 2015). For the sake of environmentally friendly marine management, it is of utmost importance to distinguish desirable from undesirable impacts and to take action to promote the former while at the same time mitigating the latter. To that end, a proper understanding of mechanisms behind the impacts is needed (Dannheim et al., 2020) in order to develop effective nature-inclusive designs that are, for example, mandatory for the development of new OWFs in the Netherlands (Ministerie van Economische Zaken, 2019). Requirements may include eco-designing scour protection layers to enhance fish habitat or restore oyster beds (Glarou et al., 2020) and deploying add-on structures such as fish hotels (Hermans et al., 2020). To avoid contributing to ocean sprawl, the use of add-on structures (i.e., artificial structures away from the turbines) may be questionable and deemed undesirable (Firth et al., 2020). The present proliferation of nature-inclusive designs will undoubtedly add new challenges to the decommissioning debate. For example, when commercial fish stocks are proven to benefit from OWFs, will these positive effects then be nullified when OWFs are decommissioned?
ACKNOWLEDGMENTS
The authors want to thank Y. Laurent (RBINS) for manuscript preparation. This paper contributes to the FaCE-It and PERSUADE projects financed by the Belgian Science Policy Office, and the Belgian WinMon.BE offshore wind farm environmental monitoring program. Joop Coolen was funded by NWO Domain Applied and Engineering Sciences under grant 14494.






