INTRODUCTION
Offshore wind has proven to be a valuable source of clean energy, particularly in Europe, where over 75% of the global capacity is installed (GWEC, 2019). In 2019, China and the United States were the greatest contributors of new wind installations (onshore and offshore combined), and with 15 offshore leases, the United States has potential as a strong contributor to the future offshore wind industry (BOEM, 2019; GWEC, 2019).
Located 4.5 km from Block Island, Rhode Island, the Block Island Wind Farm (BIWF) is the first commercial offshore wind farm (OWF) in the United States. The BIWF consists of five jacket-foundation turbines (150 m tall, 15,000 tons, 150 m rotor diameter, 30 MW total capacity) spaced approximately 1 km apart. The foundations were installed by mid-2015, and the facility became operational in late 2016, primarily supplying power to Block Island, with excess power transmitted to the mainland via a 34 km subsea export cable (HDR, 2019).
To understand the environmental effects of OWFs, the US Department of the Interior Bureau of Ocean Energy Management (BOEM) initiated the Real-Time Opportunity for Development Environmental Observations (RODEO) program in 2015. Thus, evaluation of the effects of early OWFs can inform management about how to avoid or mitigate impacts of future facilities and how to prioritize future monitoring efforts. The BIWF provided the first opportunity in the United States to evaluate the intensity, duration, and spatial scale of perceived impacts. During the construction and/or operational phases, assessments of sediment disturbances, sound emissions, visual disturbances, and effects on the benthic environment were made (e.g., HDR, 2019, 2020a,b). Here, we focus on the RODEO benthic monitoring effort during the initial operational phase and report on the effects of the BIWF on benthic ecology within four years post-construction (late 2016 to late 2019). This relatively short-term monitoring aimed to evaluate near-field spatiotemporal changes in sediment grain size, organic enrichment, and benthic macrofauna due to the presence of the BIWF foundations. This effort later expanded to evaluate the benthic changes occurring closer to and under the foundation structures and the colonizing community on the structures.
We first provide a contextual overview of benthic ecology and related fish patterns in the broader area of Block Island Sound (BIS). We then briefly describe the RODEO benthic monitoring effort at the BIWF and highlight benthic changes observed. These changes and their potential ecological importance are discussed with respect to their cascading effects and relevance to managed species. The overarching lessons learned from the implementation of the RODEO benthic monitoring effort provide insights that can guide recommendations for future efforts. We conclude by drawing parallels with European OWF environmental monitoring regimes, providing possible paths forward for future US OWF monitoring efforts.
BENTHIC ECOLOGY OF BLOCK ISLAND SOUND
BIS is an ecologically and socioeconomically important area, and to help select an appropriate site for the BIWF, the Rhode Island Ocean Special Area Management Plan (OSAMP; CRMC, 2010) was developed. As part of this multidisciplinary effort, the benthic ecology, habitats, and fishery resources of BIS and Rhode Island Sound (RIS) were characterized (Malek et al., 2010; LaFrance et al., 2014). We briefly review the knowledge gained, focusing on the benthic ecology and demersal fish of BIS to provide context for the broader BIS area prior to the BIWF.
The pre-siting OSAMP study mapped benthic habitats within a 138.6 km2 area of BIS (LaFrance et al., 2014). Water depth in this area ranges from 13–44 m. The seafloor was found to be a heterogeneous environment, consisting of five glacial depositional environment types (moraine shelf, inner shelf moraine, delta plain, alluvial fan, lake floor basin) and a range of seabed types (flat/featureless areas, sheet sands, sand waves, small dunes, boulder fields). The area was generally described as a coarse sediment environment with medium to very coarse sands dominating, though areas of finer sediments were recorded. Generally, benthic macrofauna communities were dominated by amphipods, polychaetes, and bivalves. The macrofauna community composition in BIS was influenced by mean water depth, benthic surface roughness, geological features, and sediment types at fine and/or broad scale resolutions (LaFrance et al., 2014). The Coastal and Marine Ecological Classification Standard (CMECS; the US standard) was used to describe biological and physical characteristics and to define habitats referred to as biotopes (FGDC, 2012). Within the OSAMP BIS study area, 12 distinct biotopes were identified (Figure 1, which also identifies the BIWF site; LaFrance et al., 2014).

Figure 1. Benthic biotopes within the Block Island Sound study area (138.6 km2). Twelve biotopes were identified during preparation of the Rhode Island Ocean Special Area Management Plan (LaFrance et al., 2014). The inset, with turbine positions shown, delineates the Block Island Wind Farm (BIWF) study area for the benthic monitoring that took place as part of the US Department of the Interior Bureau of Ocean Energy Management monitoring program called Real-Time Opportunity for Development Environmental Observations (RODEO). > High res figure
|
The pre-siting OSAMP study also highlighted that the benthic habitat heterogeneity and associated prey species played a role in driving demersal fish patterns (Malek et al., 2010; Kritzer et al., 2016). Both the demersal fish assemblage and stomach contents of fish were dependent on the geographical location and benthic habitat where fish were caught (Malek et al., 2010). Compared to the neighboring RIS, BIS had lower fish species abundance and biomass possibly due to lower primary production; however, BIS had greater species diversity, likely due to greater habitat complexity (Malek et al., 2010; Nixon et al., 2010). In addition to benthic habitat heterogeneity, the demersal fish community was influenced by water depth (Malek et al., 2010). Generally, communities with more even species distribution and greater abundance and biomass were found in deeper waters, while lower density yet more diverse communities occurred in shallow waters. Overall, the heterogeneous benthic habitats of BIS support a rich diversity of fish species important to both recreational and commercial fishing communities (Malek et al., 2010).
POST-CONSTRUCTION RODEO MONITORING STRATEGY AT THE BIWF
The Monitoring Effort
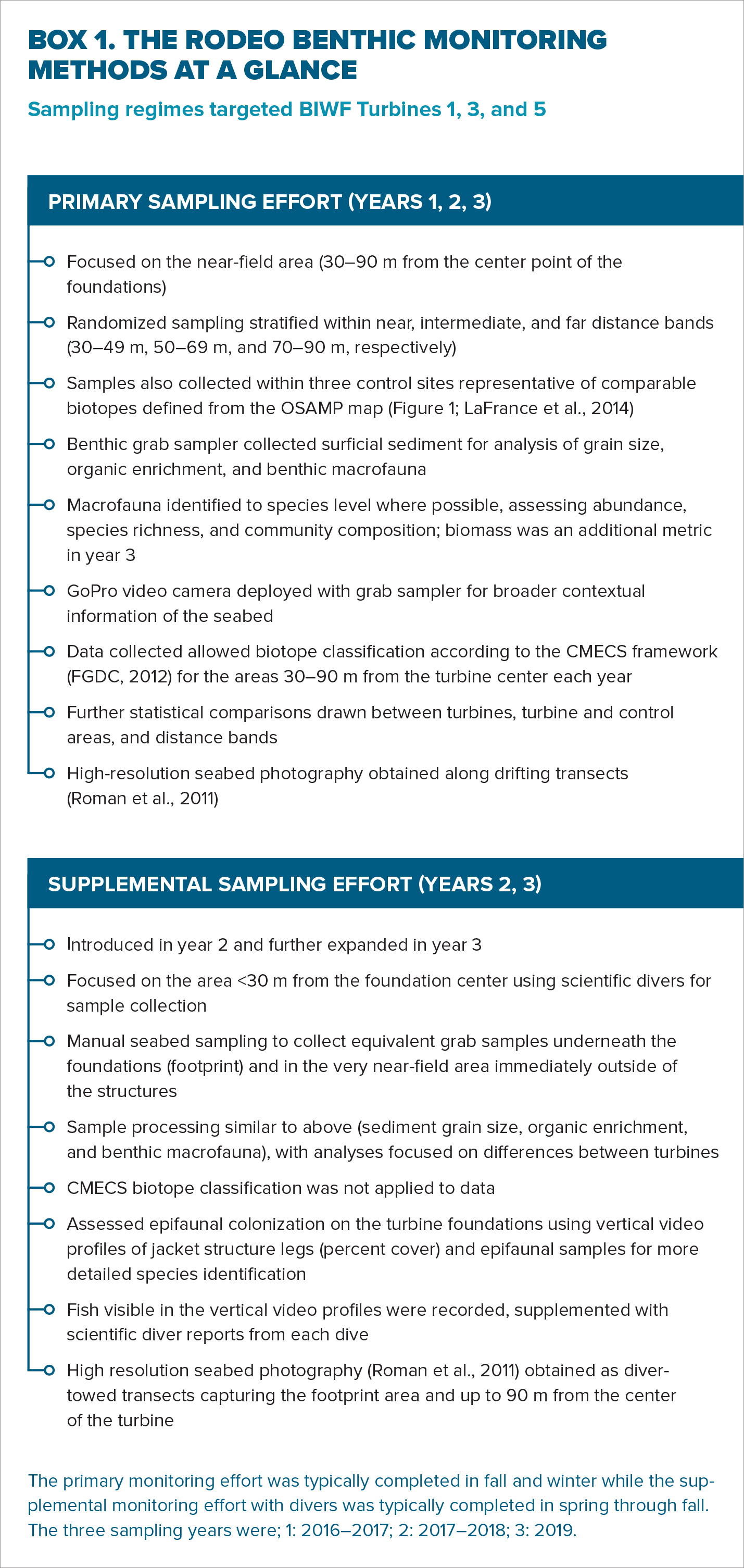
The RODEO benthic monitoring program was completed over three sampling years spanning four calendar years after the BIWF foundations were installed (from late 2016 to late 2019). This program was initially designed in 2015 based on strategies and key findings from monitoring programs and studies in Europe. At that time, there was some evidence of sediment fining, organic enrichment, and benthic macrofaunal changes close (<15 m) to gravity devices and monopiles (Wilhelmsson et al., 2006; Coates et al., 2014). However, information regarding effects surrounding jacket structures was more limited (Schröder et al., 2006; Krone et al., 2013). Observations of epifouling on gravity, monopile, and jacket turbine foundations (Schröder et al., 2006; Emu Limited., 2008; De Mesel et al., 2015) suggested considerable quantities of additional biomass could be introduced to offshore areas. Foundations were proposed as biomass “hotspots” with potentially high exports to local areas (Krone et al., 2013). Additionally, benthic ecological changes linked to enrichment effects were well documented around some fixed oil and gas structures in the United States and Europe (Wolfson et al., 1979; Page et al., 1999; Manoukian et al., 2010), although these structures are much larger.
The RODEO benthic monitoring program therefore originally aimed to detect the presence of any measurable close-range spatiotemporal differences in sediment composition, organic content, and/or benthic macrofaunal communities (HDR, 2020a). The primary sampling effort was later supplemented with data collection using scientific divers in years 2 and 3 (Box 1) to allow further benthic data collection closer to and under the structures, as well as characterization of the community colonizing the structures. Overall, the monitoring program was iterative in its design and expanded to examine aspects of the three-dimensional benthic effects that OWFs have on the ecosystem.
The primary data, collected within 30–90 m of each of the three turbines targeted for sampling (Box 1), were used to classify benthic biotopes according to CMECS (FGDC, 2012). The geological environments defined by the OSAMP (LaFrance et al., 2014) have been stable over space and time, and accurate within tens of meters, and therefore were used to define biotope boundaries for the RODEO study (Steimle, 1982; LaFrance et al., 2014; HDR, 2020a). The methods informing the CMECS classification and subsequent analyses were identical to the OSAMP study and were repeated each RODEO sampling year. While the sampling regimes for the BIWF and BIS study areas were conducted on different spatial scales (site-specific versus regional, respectively), subjecting the data to identical analyses and using CMECS as a common language permitted comparisons to be made. Additionally, direct comparisons between years of the RODEO monitoring data provide insight on the local spatiotemporal changes occurring as a result of the BIWF.
|
Benthic Changes at the BIWF
The greatest benthic changes have occurred on or within the footprints of the jacket structures four years post-installation. HDR (2020a) provides a full account of the results.
All submerged parts of the foundation structures studied were colonized by epifauna dominated by the blue mussel (Mytilus edulis) along their full vertical extents (Figure 2). Other epifauna species were present in comparatively lower coverage, including hydroids, algae, sponges, and anemones such as Metridium senile. Additional species identified and common to the region included the widespread nonindigenous invasive tunicate Didemnum vexillum and the indigenous coral Astrangia poculata (Valentine et al., 2009; Grace, 2017). Within the footprint of Turbine 1, mussel aggregations estimated to be up to 50 cm deep developed on the seabed and foundation grate, while aggregations within Turbines 3 and 5 exhibited lesser spatial coverage and density and took longer to appear (Figure 3). Multiple abundant predators associated with the mussel communities included moon snails (Naticidae), crabs (Cancer sp.), and sea stars (Asterias forbesi).
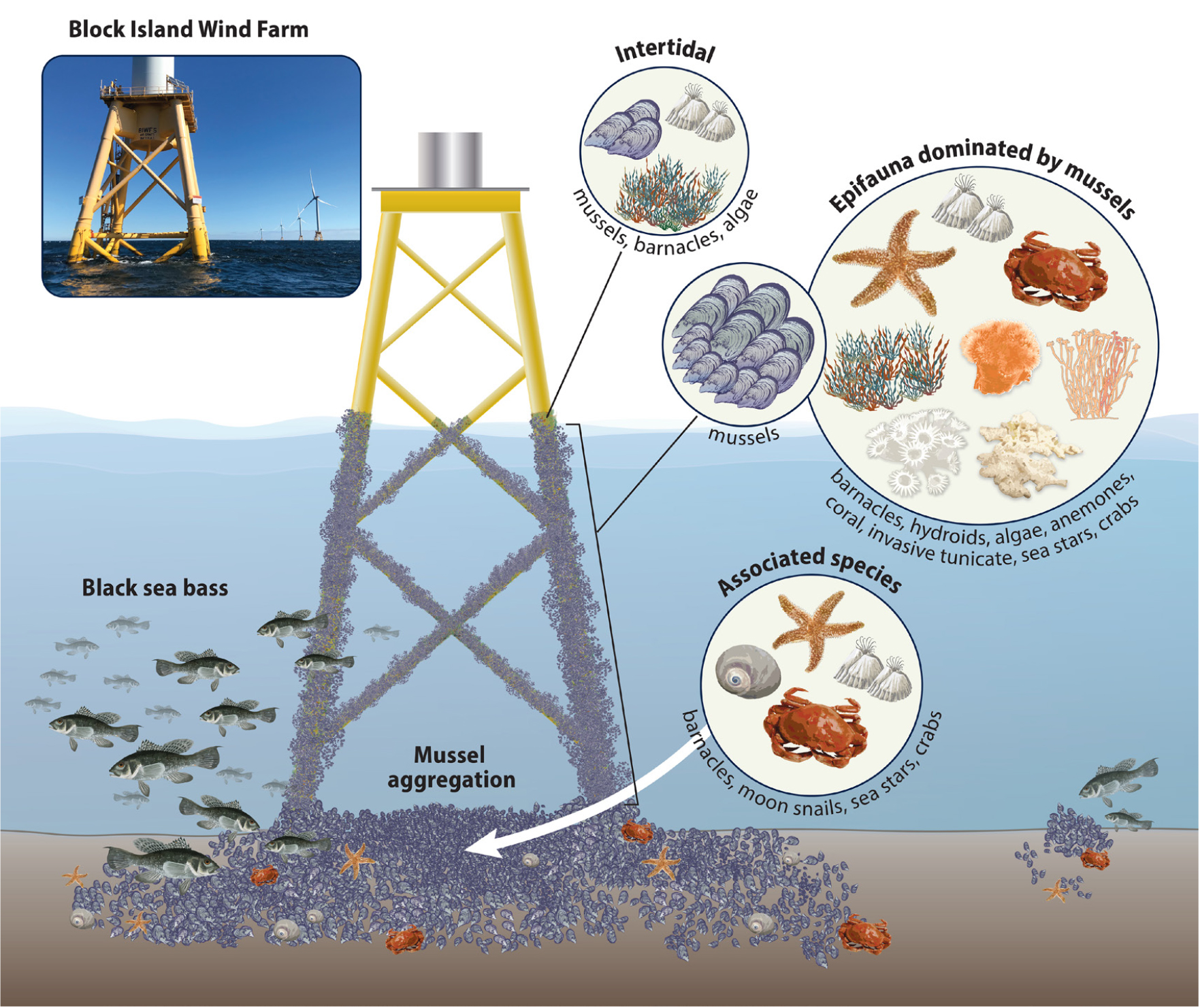
Figure 2. Fauna associated with the Block Island Wind Farm jacket structures four years post-installation. The jacket structures were dominated by filter-feeding mussels and associated epibionts. Mussel aggregations dominated the footprint of the jacket structures. Predators such as sea stars, moon snails, and crabs, as well as numerous fish had become attracted to the structure and associated epifauna. From HDR (2020a). > High res figure
|
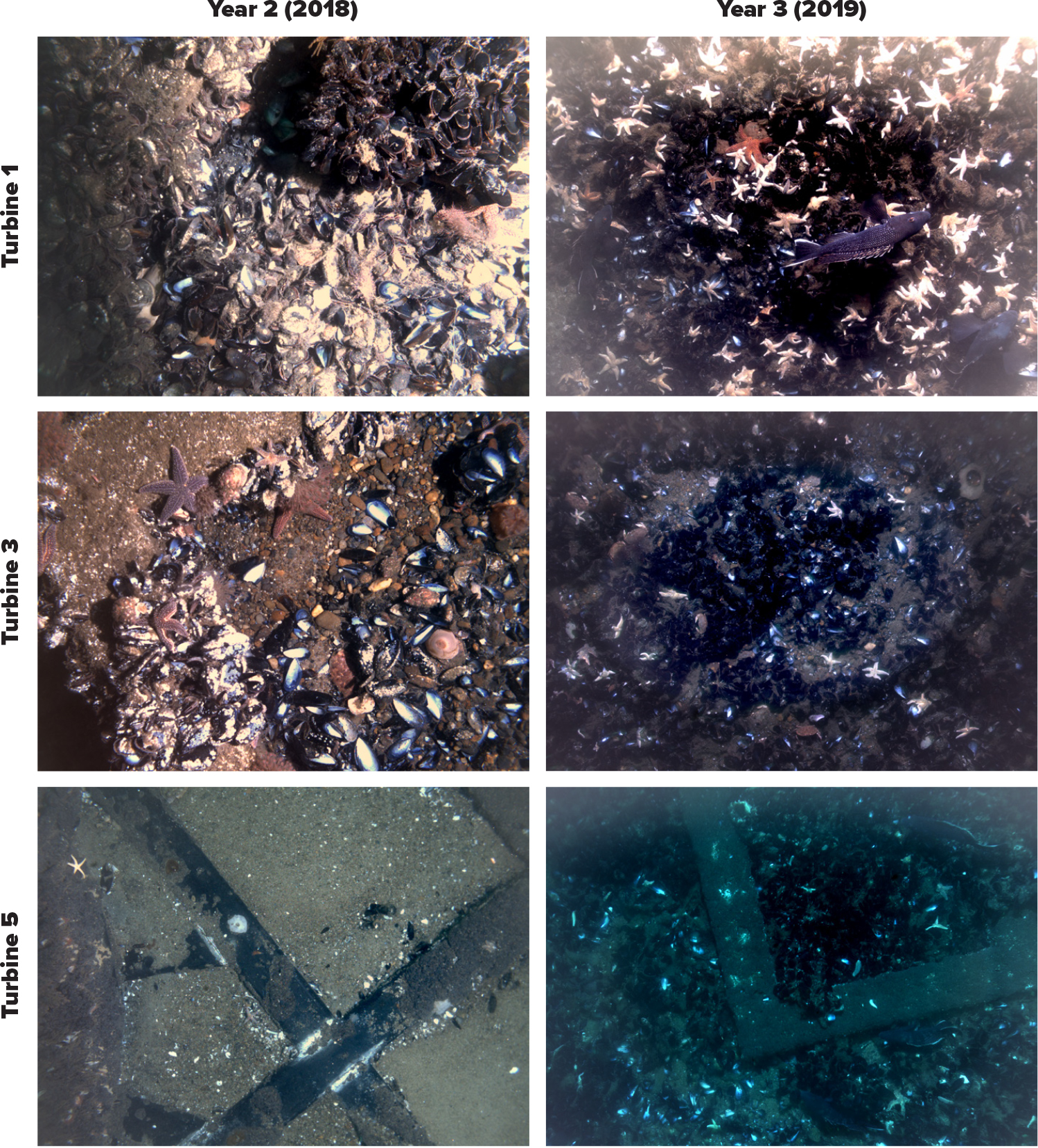
Figure 3. Benthic macrofauna within the footprint of the BIWF jacket structures in years 2 and 3. Mussel aggregations that had fully covered the footprint of the foundation of Turbine 1 by year 2 (seabed and grate) intensified by year 3 to aggregations 35–50 cm thick. Changes at Turbines 3 and 5 occurred over a longer timeframe. At Turbine 3, patchy aggregations of mussels developed within the footprint in year 2, while at Turbine 5, none were present, and the grate was fully exposed. By year 3, aggregations at Turbines 3 and 5 resembled earlier aggregations at Turbine 1. Numerous predators (crabs, sea stars, moon snails) were found in association with the mussel aggregations. From HDR (2020a). > High res figure
|
Over time, there was also a notable increase in black sea bass (Centropristis striata) around the structures, estimated to exceed 100 individuals per turbine in year 3 (Figure 4). Scientific divers also reported the frequent presence of Atlantic striped bass (Morone saxatilis) schooling at the base of the turbines, bluefish (Pomatomus saltatrix) observed in midwater around the turbines, scup (Stenotomus chrysops) at the base of the structures, and occasional schools of dogfish (Squalus acanthias). In addition, rock gunnels (Pholis gunnellus) made use of the mussel habitat, and a monkfish (Lophius americanus) was resident at one of the turbines.

Figure 4. Fish presence at the BIWF. Black sea bass (Centropristis striata) dominated the video footage of the colonized BIWF structures four years post-construction. The base of Turbine 3 is shown here. From HDR (2020a). > High res figure
|
Within the 30–90 m distance bands, no strong gradients of change in sediment grain size, enrichment, or benthic macrofauna were observed at the turbines investigated. Within this area, Turbines 3 and 5 had the most stable biotopes, dominated by polychaetes (Figure 5). Across all three sampling years, the Turbine 3 biotope was classified as Polycirrus spp. in coarse sand with small dunes within glacial alluvial fan. The Turbine 5 study area was the most heterogeneous, with three different biotopes. One biotope, Polycirrus spp., in pebble, gravel, and coarse sand within moraine shelf, remained stable over the study period. A second biotope characterized by Polygordius spp. in coarse sand with small dunes/sand waves within moraine shelf in years 1 and 2 became dominated by Polycirrus spp. in year 3. The third biotope was co-dominated by Polycirrus spp. and Lumbrineris spp. in coarse sand with small dunes within glacial alluvial fan in year 1. In year 2, the co-dominant species changed to Parapionosyllis longicirrata, Polycirrus spp., and Pisione spp., but in year 3 Polycirrus spp. dominated.

Figure 5. Change in dominant biota within biotopes over time at the BIWF. The 30–90 m areas around the three turbines were reclassified each year using the Coastal and Marine Ecological Classification Standard (CMECS) framework. Change in dominant species is highlighted from the OSAMP and RODEO monitoring effort. Note the strongest change occurs within the Turbine 1 biotope in year 3, now dominated by filter feeders Balanus spp. and Mytilus edulis. From HDR (2020a). > High res figure
|
Comparatively, by year 3, the biotope at Turbine 1 exhibited substantial change. Initially, the biotope was characterized by the polychaete Sabellaria vulgaris in coarse sand with small dunes within a glacial alluvial fan. In year 2, it was dominated by Polygordius sp., which had been abundant in year 1. The change in dominance was attributed to the patchy distribution of S. vulgaris in year 1 rather than turbine-related changes. In year 3, however, the biotope exhibited a stark change in characterizing species, biological traits, and function. Although polychaetes remained in the community composition, the biotope became co-dominated by Balanus spp. (barnacles) and M. edulis. These species were also dominant in communities found on and under the jacket structures, and so this change in biotope was strongly associated with the presence of the colonized foundation structures. Furthermore, this new biotope had not previously been recorded in the broader BIS area (Figure 1).
Turbine 1 differed from the other turbines with respect to proportions of epifaunal coverage on the structure, the extent of mussel aggregations within the footprint (Figure 3), and the shift in dominant species and resultant biotope classification. Temporal trends suggest that Turbines 3 and 5 are undergoing similar changes to Turbine 1 but at a slower pace. A gradient in benthic species composition reflects the geography of Turbines 1 through 3 and 5. This spatiotemporal gradient was also observed in the abundance of organisms, particularly for M. edulis within the footprint of the turbines. Although depth was identified as an influential factor for benthic macrofauna composition in the BIS area, there may be other related factors (LaFrance et al., 2014), such as bottom current strength and degree of wind-induced hydrodynamic disturbance. Multibeam data showed bedform features (e.g., sand ripples) at Turbines 3 and 5, but none at Turbine 1, which is located in the deepest water (30 m) (HDR, 2020b). Additionally, construction marks were persistent at Turbine 1 but not at Turbines 3 and 5 (HDR, 2020b). While the hydrodynamics were not measured, these observations suggests that the seabed and parts of the Turbine 1 structure may be exposed to lower hydrodynamic energy compared to Turbines 3 and 5, which may partially explain the more rapid successional and benthic changes at Turbine 1. Collectively, spatiotemporal changes within the BIWF indicate within-array heterogeneity that has also been observed within some European OWFs (Lefaible et al., 2019).
ECOLOGICAL IMPORTANCE OF BENTHIC CHANGES AT THE BIWF
The Benthos and Cascading Effects
As reported for other OWFs (Dannheim et al., 2020), the BIWF structures were quickly colonized and increased local diversity through increased habitat complexity (i.e., the provision of new habitat). Structurally, the BIWF provided vertical and horizontal hard substrate to be colonized in an otherwise coarse sand environment (LaFrance et al., 2014). The strong vertical epifaunal zonation observed on European foundation structures (Krone et al., 2013; De Mesel et al., 2015) was not observed at the BIWF, suggesting that four years post-construction, the colonizing community may still be in an intermediary successional stage (Kerckhof et al., 2010). It is possible that zonation on jacket structures such as the BIWF may differ from monopile and gravity structures, with mussels extending farther down the vertical profile (Krone et al., 2013). However, similar properties of a biomass hotspot (Krone et al., 2013) were recorded at the BIWF, and the benthic predators (snails, sea stars, crabs) present on and under the structures were likely benefiting from the new prey communities. Additionally, based on the presence of juvenile crabs (Cancer sp.), the BIWF potentially serves as a nursery ground, as suggested from increased production rates for crabs (Cancer pagurus) at European OWFs (Krone et al., 2017). The dominant mussel community created three-dimensional habitat complexity on an otherwise smooth structure, benefiting small reef species such as cunner (Tautogolabrus adspersus), while at a larger scale, the turbine structures hosted abundant black sea bass (C. striata) and other indigenous bentho-pelagic fish.
Functionally, the highly abundant mussel population on and within the structures’ footprints will change the local ecosystem processes, including high filtration rates of local phytoplankton, increased excretions to the surrounding seabed (Maar et al., 2009), and increased carbon assimilation, particularly by M. edulis (Mavraki et al., 2020). By year 3 there was clear evidence of the mussel populations extending beyond the BIWF structures (30–90 m). The change in biotope classification around Turbine 1, resulting from the change in dominant species, demonstrates a shift in biological traits and function in the surrounding area related to the presence of the colonized turbine structure (Figure 4). While there were some biotope changes at Turbines 3 and 5, the biota remained dominated by polychaetes, which are deposit- and filter-feeding, burrowing, or tube-building or burrowing bioturbators (Hutchings, 1998). Comparatively, the dominant biota of the Turbine 1 biotope were barnacles and mussels, which are sessile filter feeders, and encrusting or bed-forming species, which offer sediment consolidation (Trager et al., 1990; Riisgård et al., 2011; Fariñas-Franco et al., 2014), while the supporting polychaete community contributes bioturbation in the local area.
The bioengineering properties of mussels were evident within the turbine footprints and within the new biotope at select sample locations. Patches of adult mussels with associated fine sediments, organic enrichment, and modified benthic macrofaunal communities were recorded within 50 m of Turbine 1. Similar patches of mussel and associated properties near turbines (~37.5 m) were recently recorded within the Thornton Bank Belgian OWF and were proposed to be the result of adult mussels transported from the structures (Lefaible et al., 2019).
High mussel exports are expected (Krone et al., 2013), although it is likely that the off-structure mussel aggregations at the BIWF are not only mussels that dropped off of the structures but also new recruits. The high abundance of juveniles in the year 3 sampling indicates local spat settlement and suggests suitable conditions for an expanding population. Mussels have already been found in areas further from the BIWF, beyond the spatial scope of the RODEO effort, 1.6–4.8 km west of Turbine 5, where they were not previously recorded (Wilber et al., 2020). The addition of the BIWF mussel population and other epibionts could have far-reaching larval distributions—tens of kilometers—increasing connectivity between natural and OWF populations (Gilg and Hilbish, 2003; Coolen et al., 2020). The contribution of larval connectivity to further proliferation of filter-feeding populations may then influence carbon cycling at broader geographical scales. Models indicate that the increased population of filter feeders resulting from OWF proliferation in the southern North Sea Basin may lead to regional changes in primary productivity (Slavik et al., 2019), but as yet there are no comparable modeled scenarios incorporating the OWF expansion along the US East Coast.
Potential Importance to Managed Species
OWF structures may have direct and indirect effects for some species, especially when they are situated where hard substrates and associated epifauna are scarce. These effects may be particularly important for managed species, those for which management plans have been developed because they are economically or culturally important or because of their population status (e.g., small, declining, or dependent on vulnerable habitats). The OWF artificial reef effect is now relatively well characterized as benefiting fish and shellfish by providing refuge and creating forage, and as attracting abundant and diverse communities, although some processes require further attention (Degraer et al., 2020, in this issue).
Recent meta-analysis of finfish within European OWFs highlights a broadly positive effect on fish abundance during the operational phase (Methratta and Dardick, 2019). Examples of increased abundance and biomass of culturally important species include Atlantic cod (Gadus morhua), pollock (Pollachius pollachius), pout whiting (Trisopterus luscus), and crabs (Cancer sp.) (Wilhelmsson et al., 2006; Bergström et al., 2013; Reubens et al., 2014; Krone et al., 2017). Atlantic cod and pout whiting aggregate around OWF foundations in the North Sea in response to increased food availability provided by colonizing species (Reubens et al., 2014; Mavraki, 2020). Metabolic analyses of both species indicate sufficient energy for growth, suggesting localized increased fish productivity, but no evidence of regional effects have been documented (Reubens et al., 2014). Furthermore, the degree of attraction was found to vary seasonally, highlighting the importance of incorporating species life history and movement ecology in any monitoring efforts.
Trophic and energetic analyses of fish around the BIWF structures have yet to be conducted; however, nearby, increased findings of mussels in the stomachs of winter flounder (Pseudopleuronectes americanus) have been recorded (Wilber et al., 2020). Changes in primary productivity due to increased filter-feeding populations (Slavik et al., 2019) may also become important for planktivorous fish in BIS (Malek et al., 2010) and future OWF areas. Seasonality in fish use of the BIWF has also yet to be addressed, although the presence of fish suggests they are profiting from the provision of food and/or shelter. Black sea bass (Figure 4) and other structure-oriented species will likely benefit from future US OWFs. Local fishers have targeted large tautog (Tautoga onitis) near BIWF foundations and noted that Atlantic cod are attracted to the area (ten Brink and Dalton, 2018), consistent with data from European OWFs (Reubens et al., 2014). Whether fish resources increase (i.e., production) around OWFs, or biomass is simply redistributed (i.e., aggregation) requires clarification. Recent evidence demonstrates energy savings in juvenile Atlantic cod associated with stone reef habitats compared to sand habitats, which may allow energy to be allocated to growth and thus increase production (Schwartzbach et al., 2020). Similar studies of metabolic rates of cod and other species associated with OWFs and comparable local habitats would be beneficial going forward.
Managers must also consider the value of habitat change (Gill, 2005). The OWF reefs differ from natural hard substrates and cannot be considered a substitute (Kerckhof et al., 2017), although they may have added value, albeit different value (Degraer et al., 2020, in this issue). The new structural habitat gained exceeds the seabed habitat lost in terms of spatial extent. However, the transition from natural soft-bottom substrate to hard substrate habitat (including mussel-dominated biotopes) may displace species that prefer soft-bottom habitats and associated prey. Prior to construction, the BIWF area was mostly coarse sand with some pebble and gravel substrate, essential fish habitat for 24 managed species (CRMC, 2010). To fully determine the value of change in habitat as relevant to managed species, the habitat and fish need to be assessed. This likely requires using varied techniques that are applicable to selected demersal and pelagic species (e.g., demersal trawl, hook and line surveys, fyke nets, diver surveys, telemetry), targeted both at the structures and far from them over valid temporal scales. In the northwest Atlantic, several species use soft-bottom habitat slated for OWFs, including numerous flatfish and longfin inshore squid (Doryteuthis pealeii), an important prey species (Jacobson, 2005). Lower impacts and quicker recovery from disturbances in soft bottoms (e.g., mud, sand) have been found (Grabowski et al., 2014), but the number of species affected or their ecosystem values may be larger (Henriques et al., 2014; Kritzer et al., 2016). To date, the majority of OWFs have resulted in the introduction of hard structure into a soft sediment environment. However, the United States has leased an area in natural, complex, hard-bottom habitat that provides important ecosystem functions (e.g., cod spawning ground; Zemeckis et al., 2014). While a lease in this habitat type and the associated habitat change are atypical, the ecological significance will need to be defined.
STRATEGIC LESSONS FROM BENTHIC MONITORING AT THE BLOCK ISLAND WIND FARM
The RODEO benthic monitoring philosophy involved observing and quantifying near-field changes in benthic ecology at the BIWF. It was the first opportunity to observe such changes at a US OWF, and given the BIWF’s novelty, it was reasonable to focus on small-scale spatiotemporal changes. The relatively short-term seabed sampling strategy was designed to provide insight into changes in sediment composition, organic enrichment, and benthic macrofauna. The adaptive nature of the strategy proved valuable for assessing benthic changes observed close to the structures and also expanded to incorporate artificial reef effects, providing a multi-faceted overview of changes occurring around the BIWF.
Complementary information was obtained using primary and supplemental methods (Box 1). The primary randomized benthic sampling across distance bands allowed a broad spatial view while assessing gradients of change in sediment, enrichment, and benthic macrofauna from the turbines. The application of the CMECS framework highlighted the importance of fully characterizing biotic changes and provided a sufficiently high-level of classification to allow contextualization of local biotopes within the regional pre-siting OSAMP biotopes. Future studies will need to carefully consider appropriate baselines (new data collection and/or pre-existing data) and their comparability to new data collected throughout the monitoring effort. A fuller understanding of the benthic changes observed, including a better overview of gradients of change with proximity to the turbines, was obtained from the supplemental sampling. However, the integration of primary and supplemental data sets was challenging, and further attention to the comparability of varied sampling strategies or addition of relevant controls may benefit future monitoring efforts. Longer-term monitoring would be required to determine the climax colonizing community on the structures and the potential development of vertical zonation. Future monitoring efforts should also consider seasonal effects in biota and use targeted fish surveys to quantify abundance, community composition, and the relevance of artificial reef effects to local species (e.g., quantifying trophic or refuge effects on fish biomass). Where grab sampling is prevented due to mussel aggregations, the incorporation of reef metrics may be useful (e.g., Hendrick and Foster-Smith, 2006; Gubbay et al., 2007). Finally, the development of automated methods may allow rapid characterization from benthic photography. The appropriate application or development of refined methods should be based on strategic prioritization of the ecological questions to be addressed.
The RODEO benthic monitoring effort provides regionally relevant (i.e., for the US East Coast) observations of benthic changes similar to those observed from European and UK OWFs and establishes a platform from which to prioritize future US monitoring efforts. Going forward, our recommendation is to move beyond the philosophy of this monitoring effort to focusing on gaining a deeper understanding of the effects of OWFs on benthic ecology and their functional relevance to the broader ecosystem. Benthic ecology plays a vital role in trophic provisions, biogeochemical processes, and biodiversity (Dannheim et al., 2020). Addressing how OWFs affect these functions will require careful collection of empirical data at spatiotemporally relevant scales as well as modeling in order to understand regional importance (Wilding et al., 2017). Consideration of the functional changes over the life of an OWF will require data collection and modeling over a longer timeframe and a broader spatial scale, partnered with suitable long-term pre-OWF comparisons, and further should incorporate analyses of cumulative effects (Wilding et al., 2017; Willsteed et al., 2017).
ENVIRONMENTAL MONITORING—THE PATH FORWARD
The ecological lessons learned from the BIWF benthic monitoring program—for example, observations of rapid colonization dominated by filter feeders and attraction of fish as well as indigenous and non-indigenous species—are largely similar to observations in European OWFs (Degraer et al., 2020, in this issue). We acknowledge that there are apparent differences; the species (including their value and health) are region specific, the hydrodynamic systems are different (e.g., open coast, basin), and there may be some influence from foundation types. However, the effects on the functioning of the ecosystem are largely comparable. Consequently, the underlying scientific questions appear broadly applicable, offering great opportunities for enhanced efficiency and effectiveness of monitoring programs as OWFs proliferate along the US coasts. Rather than designing monitoring programs on a project-by-project basis, resource efficiency implies that monitoring programs should ideally focus on a careful selection of OWFs considered representative of a region, or suitable for a given research question. This approach is exemplified by basic and targeted monitoring (Lindeboom et al., 2015), as adopted by the Belgian WinMon.BE monitoring program that encompasses eight OWFs (Degraer et al., 2009; Figure 6).
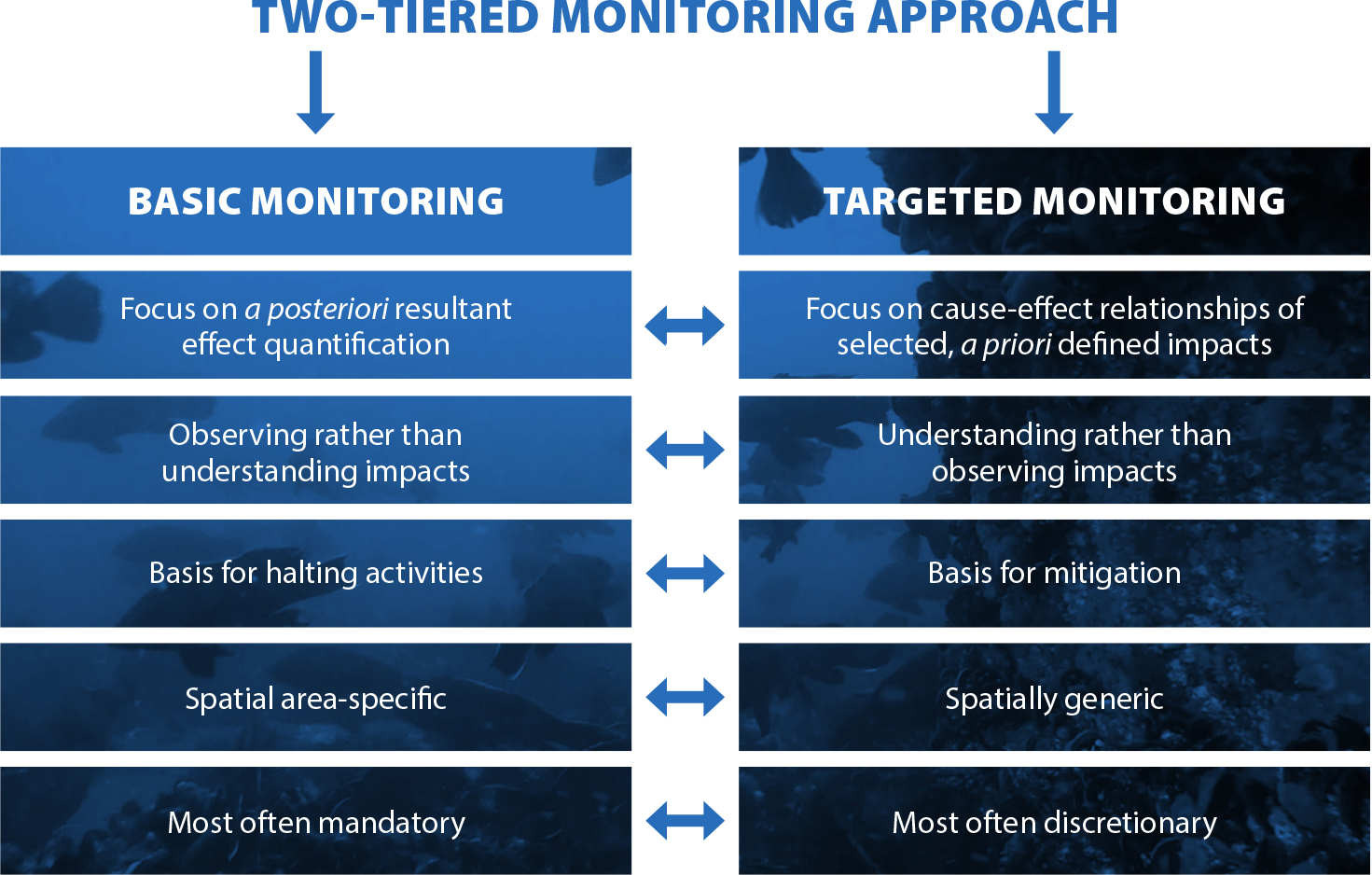
Figure 6. Recommendations for future monitoring approaches. A two-tiered monitoring approach embracing basic monitoring and targeted research allows for a comprehensive overview and understanding of impacts, maximizing the value of the monitoring results. > High res figure
|
Basic monitoring aims to objectively evaluate impacts of OWFs a posteriori, allowing even unforeseen impacts to be evaluated. Targeted monitoring aims to understand the underlying ecological processes behind the prioritized observed impacts. It allows results to be extrapolated beyond the study area to enhance impact prediction and evidence-based mitigation. Basic monitoring is usually mandatory, integrated within OWF environmental permitting, whereas targeted monitoring is often dependent on governmental research funds.
Industry manages most monitoring programs in Europe, informed by national environmental permit and monitoring standards (e.g., the StUK4 monitoring scheme in Germany; BSH, 2013). These permit-based monitoring programs neither allow the flexibility needed to adopt basic and targeted monitoring schemes nor optimize programs as new scientific knowledge becomes available. Furthermore, data from industry-managed monitoring programs are generally not publicly accessible and often only offer short-term data series that result in information of limited value.
To remedy these issues, Belgium has opted for a single, integrated, public-authority-driven OWF environmental monitoring program. Active since 2005, WinMon.BE facilitates an adaptive approach for basic and targeted monitoring (Degraer et al., 2019). The public authority has the explicit right and duty to share the monitoring data (UNECE, 1998) and to adapt the monitoring program according to new scientific insights. Funding is provided by financial contributions from all OWF owners as required by the environmental license. On the other hand, the public-authority-funded Dutch Governmental Offshore Wind Ecological Programme (WOZEP), which has been active since 2016, does not cover the monitoring of specific projects but rather seeks to address the main knowledge gaps related to OWF environmental impacts, including cumulative effects (WOZEP, 2016). Both the WinMon.BE and WOZEP monitoring schemes have been noted as best practices. Situated on the cusp of extensive OWF construction in a vast marine area within the boundaries of a single country, the United States should now consider implementing a similarly coordinated monitoring strategy to allow an efficient, adaptive, combined basic and targeted monitoring scheme.
ACKNOWLEDGMENTS
Study concepts, oversight, and funding for the RODEO Program were provided by the US Department of the Interior, Bureau of Ocean Energy Management, Environmental Studies Program, Washington, DC, under HDR’s IDIQ Contract No. M15PC00002. This paper contributes to the Belgian WinMon.BE offshore wind farm environmental monitoring program. We thank Kristen Ampela, Nancy Jepsen, and Dorothy Bungert (HDR) for editing and graphics support. We also thank the guest editors for the invitation to contribute to this special issue.









