View Issue TOC
Volume 38, No. 2
Pages 52 - 57
FEATURE ARTICLE • Exploring Climate Change, Geopolitics, Marine Archeology, and Ecology in the Arctic Ocean Through Wood-Boring Bivalves
By Jørgen Berge , Torkild Bakken, Kristin Heggland, Jon-Arne Sneli, Øyvind Ødegård, Mats Ingulstad, Terje Thun, and Geir Johnsen
- Published Online: May 8, 2025
- https://doi.org/10.5670/oceanog.2025.311
- Full Article:
 PDF
PDF
- Export Article Citation: BibTeX | Reference Manager
- Share
Full Text
Background
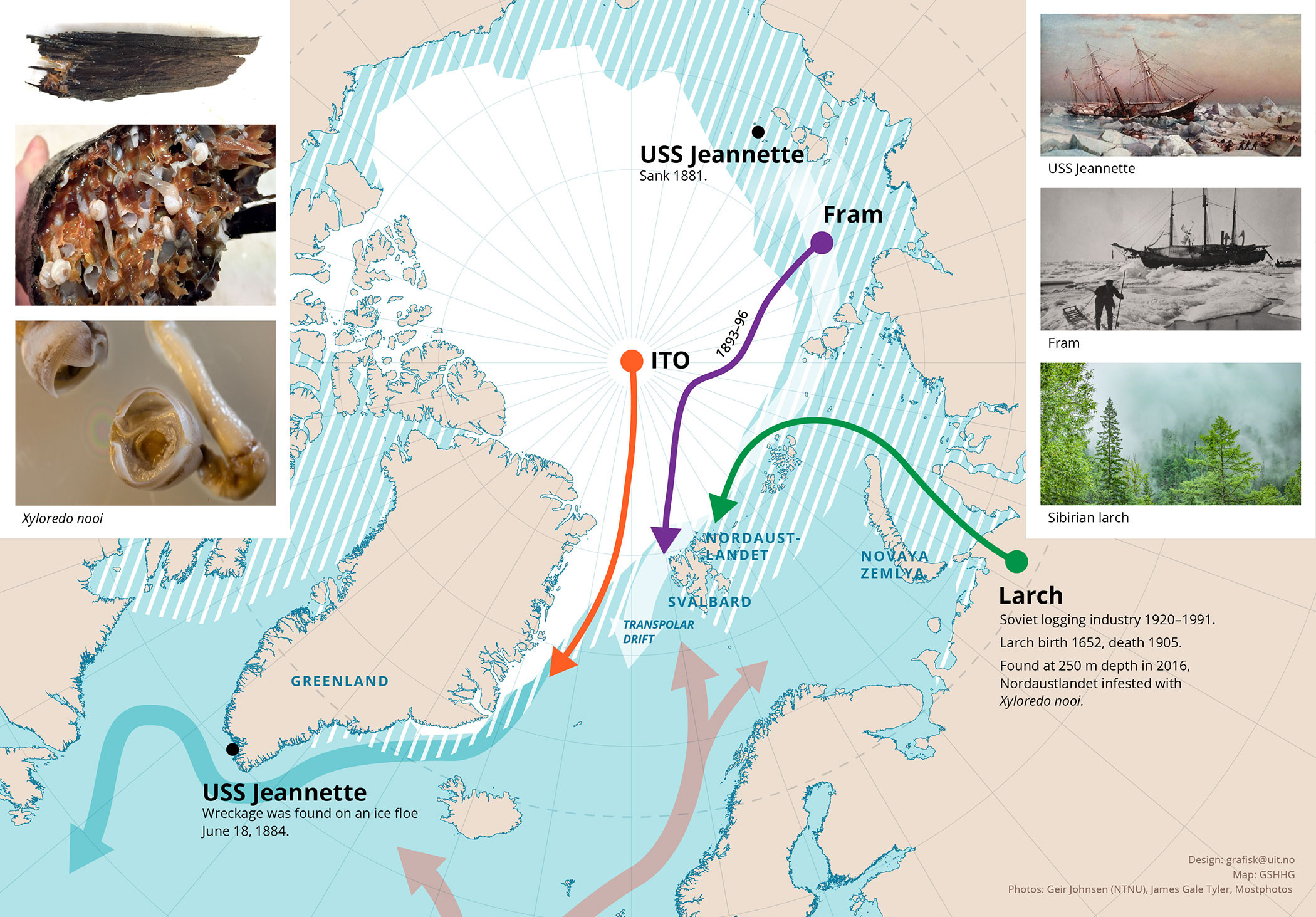
In 1652, a young Siberian larch sprouted somewhere along the Yenisei region in Siberia (Figure 1). Almost 250 years later, in 1904, that tree died. Then, in 2016, it ended up in a bottom trawl in the Arctic Archipelago of Svalbard. In order to reach Svalbard, the log must have been captured by sea ice in the Kara Sea and transported by the Transpolar Drift (TPD) across the Arctic Ocean before it was released and eventually sank in Rijpfjorden at 80°N. When it was brought up on the deck of the research vessel and examined by scientists, the log was heavily infested with living specimens of the wood-boring bivalve Xyloredo nooi. Shipworms and other wood-boring mollusks have never before been reported from the High Arctic. This is, however, not only a story about a piece of wood drifting across the Arctic Ocean and the first report of Arctic wood-boring mollusks. It also tells a story about the connection between climate and environmental conditions, and the history of human activity in Svalbard, the influence of the European timber industry and the Soviet Union planned economy, Arctic resource extraction during the last 400 years, and preservation of marine archeological artifacts—and it reveals significant gaps in knowledge concerning Arctic benthic fauna. The latter has strong implications for contemporary geopolitical issues in the region, including the ongoing debate regarding deep-sea mining.
|
|
Materials and Methods
During two research cruises on R/V Helmer Hanssen funded by The University of Tromsø – The Arctic University of Norway (UiT), pieces of wood infested with wood-boring mollusks were trapped and collected in the bottom trawl (Campelen 1800 bottom trawl) at two locations on Svalbard. In January 2016, a 7 m long log that was partly buried in anoxic sediment was collected in Rijpfjorden, located on the northern side of Nordaustlandet, Svalbard. (80°17'40.44''N, 22°18'0.07''E) at 250 m depth. A second piece of infested wood was collected on June 26, 2019, on the west coast of Svalbard in Smeerenburgfjord
at 215 m depth.
Wood-boring mollusks collected from the wood were brought back to the laboratory and identified following original descriptions of Xyloredo species (Turner, 1972). The collected specimens were found to comply with the description of X. ingolfia and deposited in the collections at the Norwegian University of Science and Technology Museum in Trondheim (NTNU-VM 82062-82067; Bakken et al., 2024). In order to undertake a thorough taxonomical identification, type specimens (paratypes) of X. ingolfia were borrowed from the Natural History Museum of Denmark in Copenhagen (NHMD-76456). The newly collected mollusks were similar to the type specimens in proportion of valves and in the sub-rectangular and well-calcified nature of an accessory plate called a mesoplax (Turner, 1972, 2002). The morphological examination did not reveal any difference between the collected specimens from Rijpfjorden and Smeerenburg. However, X. ingolfia has been synonymized with X. nooi (Voight, 2022).
Based on dendrochronology and established reference chronologies from Russian Larix, the tree ring patterns in the log collected from Rijpfjorden indicate that the tree lived during the period 1652–1904 in the Yenisei region in Siberia (Russia). For genus and species identification, methods based on morphology detailed in Kolar et al. (2022) and Alm (2019) were used for this study. For the analyses of tree rings, the CATRAS system (Computer Aided Tree ring Analyses System; see Aniol, 1983) was used. See the online supplementary material for further description of this analysis.
Results and Discussion
Two previous studies (Alm, 2019; Linderholm et al., 2021) concluded that 87% of the driftwood examined from Svalbard consisted of three genera: Pinus (pine), Picea (spruce), and Larix (larch), with Siberian larch (Larix siberica) as the dominant species. Although we cannot rule out that it is a different species of Larix, we refer here to the specimen as a Siberian larch. The exact species identification is not a key part of the findings we present, nor of the interpretation of the results. The Linderholm et al. (2021) study was carried out in the southwestern part of Spitsbergen, in a region where coastal surface currents flow northward from the southern tip of Svalbard. Irrespective of which species this is, there are no trees growing on Svalbard. Also, surface currents in our study’s part of the Arctic flow west. Hence, for a log to end up in Rijpfjorden, the only possible direction of transport is westward via the TPD (Figure 1). Thus, driftwood in Rijpfjorden will more than likely originate east of Svalbard.
The Rijpfjorden log was heavily colonized with living specimens of different sizes of the wood-boring mollusk Xyloredo nooi (Figure 1). Rijpfjorden is a north-facing fjord that has an annual extended ice cover consistently dominated by Arctic water masses (Berge et al., 2009). The bottom temperature in the region remains at –1.8°C throughout the year (Cottier et al., 2021, 2022). The second record of X. nooi was documented in the wood recovered from Smeerenburgfjord (Figure 1).
The two Svalbard fjords represent very contrasting oceanographic environments. While the north-facing Rijpfjorden is characterized by Arctic water masses, Smeerenburg, like other west-facing fjords on the main island of Spitsbergen, is strongly influenced by warm Atlantic water (Berge et al., 2005). Although there are no direct measurements of bottom temperatures in Smeerenburg, continuous measurements in Kongsfjorden, another open fjord strongly affected by Atlantic water masses just south of Smeerenburg, exhibited bottom temperatures ranging between 1.5° and 3.0°C in late June 2009 (Cottier et al., 2021, 2022). As a consequence, the fauna in the two fjords are very dissimilar, as seen, for example, in the fish fauna (Nahrgang et al., 2014; Jordà-Molina et al., 2023). Unlike in Rijpfjorden, only fragments of a log were collected in Smeerenburg, and no living specimens (just empty shells) were found. And unlike Rijpfjorden, Smeerenburg rarely freezes over, as it is strongly influenced by Atlantic water flowing northward through the Fram Strait, entering the Arctic northwest of Svalbard (Ingvaldsen et al., 2024).
Driftwood and Wood-Boring Organisms
There are two families of bivalves (Teredinidae and Xylophagaidae) that are able to settle on and digest wood or other vegetation in the marine environment. As larval stages of species belonging to these groups undergo metamorphosis, they begin to bore into and eat the wood in which they settle (Voight, 2015). Through a molecular phylogenetic study, Distel et al. (2011) found the two to be a monophyletic taxon. Many species belonging to the Xylophagaidae are poorly known, and many inhabit the deep sea. Hence, based on their common ancestry, information and status about their biology are in many cases only assumed or deducted, rather than based on detailed biological studies.
The bivalves of the Xylophagaidae occur from a few meters below low tide to more than 7,000 m depth (Turner 1972, 2002), boring into wood sunken to the seafloor using toothed ridges on their anterior shells and ingesting wood fragments (Purchon, 1941). They are considered the sole wood borers at depths greater than 200 m (Turner, 1972). A wood fall represents a massive energy input and can be compared to a whale fall on the seafloor (Ristova, et al., 2017; McClain et al., 2025). However, the energy in the wood is trapped in cellulose that most organisms are incapable of digesting. To access this energy, bottom dwellers are dependent on organisms such as X. nooi to digest the cellulose. In addition, wood-boring mollusks may also contain symbiont bacteria that enable fixation of nitrogen as well as cellulose digestion (Goodell et al., 2024). By sustaining the wood fall communities, wood-boring mollusks in the deep sea fill a role comparable to grazers in the euphotic zone (Turner, 2002; O’Connor et al., 2014; Voight, 2015).
In the Northeast Atlantic, Xyloredo is represented by X. nooi known from deep, cold waters and from deep fjord areas (Turner, 1972; Voight, 2022). A separate undescribed species was found in widespread localities in the Bay of Biscay and at the Haakon Mosby Mud Volcano in the northern Norwegian Sea (Romano et al., 2020). There is no direct evidence confirming that Xyloredo species specifically release gametes into the water column for external fertilization. Most research on shipworms in general (family Teredinidae) suggests that external fertilization is a common reproductive strategy, but this has not been explicitly confirmed for Xyloredo. Given the diversity of reproductive strategies among shipworms, such as brooding larvae internally in some species, it is possible that Xyloredo exhibits unique or unstudied reproductive adaptations. Further research is needed to clarify the reproductive biology of Xyloredo, including the mechanisms of gamete release and fertilization.
Because the size and maturity of the specimens found inside the Rijpfjorden log were distinctly heterogeneous, the demographic structure of the bivalves indicates either local recruitment and reproduction or multiple recruitment events inside the fjord. One end of the log carried clear indications of having been buried in anoxic sediments, also suggesting that the log had been partially submerged in Rijpfjorden for several years. This, and the fact that several juvenile specimens of X. nooi were found inside the log, strongly suggest local recruitment and/or reproduction. Although we cannot rule out the possibility of multiple recruitment events while the log was moving, this cannot explain the presence of juvenile specimens inside the log after several years in Rijpfjorden. As the reproductive biology of Xyloredo species remains uncertain, it is not possible to unequivocally assess how recruitment might have occurred in Rijpfjorden. Importantly, however, both possible events (or a combination of the two) challenge our status of knowledge regarding the Arctic marine benthic fauna.
Transpolar Drift
For a Siberian larch that grew in the Yenisei region until the beginning of the last century to end up in a fjord on Svalbard (Figure 1), the only mode of transport is by the TPD (Häggblom, 1982). In 1884, the Norwegian researcher and explorer Fridtjof Nansen came across newspaper reports that fragments of the hull of the steam bark Jeannette had been found on the east coast of Greenland. He knew that this ship had been frozen into the sea ice and wrecked off the New Siberian Islands three years earlier, during an attempt by the US Arctic Expedition to find entry into what was hypothesized to be an ice-free central Arctic Ocean. Nansen was inspired by Jeannette’s finding and the large quantities of driftwood from Siberia found on the shores of East Greenland, and reports of driftwood found north of Spitzbergen, and hypothesized that the Arctic Sea ice drifted westward across the Arctic Ocean from Siberia toward the Fram Strait. The existence of the TPD was later documented by Nansen’s Fram expedition in the 1890s (Nansen, 1897), as he set out to prove that the currents created by the largest Russian rivers emptying into the Arctic Ocean could push a ship across the North Pole. The TPD as mechanism to move sea ice by a combination of wind and ocean drag has been modeled to explain oceanographic surface systems in these parts of the Arctic Ocean (Spall, 2019). The importance of the TPD for the occurrence of driftwood on Svalbard has also previously been examined and documented by Eggertsson (1994). Driftwood can also archive climate information, and because the wood transported on or frozen in ice stays afloat for an extended time, it can be used to trace historical changes in currents and ice conditions (Linderholm et al., 2021). As demonstrated by a set of ice-tethered observatories (ITO) deployed at the North Pole in July 2022 (see Figure 1), the speed of the TPD has increased. Whereas it took Fram, frozen in sea ice, three years to drift across the Arctic Ocean (Figure 1), it took the ITOs deployed in June 2022 only seven months to effectively be transported out into the Fram Strait (Berge et al., 2025).
Geopolitical and Historical Context
Driftwood can be a naturally occurring and renewable resource, created by trees falling into the water due to erosion of riverbanks and the break-up of ice in the spring. However, the arrival of human settlements and industry in arctic territories also impacted the production of driftwood. The larch tree found in Rijpfjorden started its life shortly after the first Russian settlers arrived on the banks of the Yenisei, and died just as the Romanov imperial dynasty entered its last turbulent years before the Russian Revolution (1917). This occurred at a critical juncture in the development of the international timber trade in the late nineteenth and early twentieth centuries. The forests of central Europe no longer seemed inexhaustible because they could not meet the growing demand for timber from industrialization and population growth (Lotz, 2015). Thus, the timber industry frontier moved north and east, and Russia became the world’s leading timber exporter.
Commercial logging and timber rafting along the Yenisei River began in the nineteenth century. The abolition of serfdom in 1861 had increased labor mobility, and the state also encouraged settlement in Siberia. But loggers in Siberia struggled to overcome disadvantages such as lack of modern industrial equipment and transportation to the European markets. Ice conditions are difficult in rivers flowing toward the far north, and the Kara Sea was also seen as natural barrier. Only after the Finnish-Swedish explorer Nordenskiöld successfully sailed to Ob and Yenisei in 1875 did the establishment of commercial shipping routes seem feasible. Despite oceanographic research including depth soundings, hydrographic surveys, mapping of shoals and ice conditions, very few commercial shipments made it safely across the Kara Sea before 1904.
The outbreak of the Russo-Japanese war in February 1904, the same year the Siberian larch died along the Yenisei, greatly changed the strategic importance of the Northern Sea Route (also known as the Northeast Passage). The Russian Baltic fleet had to circumnavigate the world before it could reach Japan, only to be soundly defeated at the Tsushima Strait in 1905. After the war, Tsar Nicolas II launched the Arctic Ocean Hydrographic expedition (1910–1915) to open the Northern Sea Route as a strategic objective for the state (Kuksin, 1991). As a part of the new Russian commitment to expand its activities in Arctic waters, the polar explorer Rusanov sailed to Spitsbergen to take possession of coal fields and to promote Russian hunting and resource extraction, thereby strengthening the Russian position in the ongoing scramble over Svalbard.
After the Russian revolution in 1917, the new Soviet authorities sought to harness the transportation potential of the Yenisei for trade in bulky commodities (Nielsen and Okhuizen, 2022). The Soviet industrialization plans and immense appetite for wood led to intensification of Siberian logging in the 1920s and 1930s, and at the same time polar navigation techniques and technology improved. Stalinist forced-tempo industrialization, imported equipment, and skepticism toward Western approaches to sustainable management made for a massive, if wasteful, expansion of the Siberian timber industry (Kotchekova, 2024). It has been estimated that up to 50% of the timber was lost while being rafted on the Yenisei River in the early decades, providing a considerable source for driftwood in the Arctic Ocean. Over time, the share of reported losses dropped even as the transport volume increased, with the peak transport volume for the Yenisei occurring around 1960 (Hellmann et al., 2015). By that time, only 2.5% of the logs were reportedly lost during rafting (Korpachev et al., 2022). The later disintegration and collapse of the Soviet Union also affected the supply of driftwood, as harvest levels fell during the economic and political turbulence in post-Soviet Russia (Naumov, 2016).
The combination of the TPD and the logging industry in Siberia has had a significant influence on human presence and history on Svalbard. Driftwood was an important resource both for firewood and building materials. Without the extensive logging in Siberia, the total volumes of driftwood reaching Svalbard would have been far less during the last 100 years. Hence, there would have been fewer wood falls for the wood-eating bivalves, and the history of resource extraction on Svalbard would have been different. Many small cabins built by hunters and trappers using driftwood from this period still remain and are today protected as cultural heritage (Reymert and Moen, 2015).
Biodiversity and Underwater Cultural Heritage
The Svalbard fjords provide natural laboratories for exploring the effects of global warming. Fjords on the west coast receive large quantities of heat energy, organisms, and particles that are transported northward by the West Spitsbergen Current (e.g., Berge et al., 2005). Fjords on the northern part of the archipelago are more influenced by Arctic water masses. Arguably, Rijpfjorden is among the most extensively studied High Arctic marine ecosystems (e.g., Jordà-Molina, 2023), thought to host a more endemic Arctic fauna without the influence of boreal species. Finding Xyloredo nooi both in Rijpfjorden and Smeerenburg shows that significant knowledge gaps remain regarding biodiversity and distribution of species that need to be filled before we can analyze and understand how future warming of the Arctic may influence and alter biodiversity, ecosystem composition, and eventually also ecosystem services in the marine Arctic.
Moreover, and due to the fact that investigations of the few wrecks discovered in cold-water temperatures have shown no presence of wood-boring mollusks, there has been an assumption that such organisms do not thrive in the High Arctic (Stewart et al., 1995). With over 1,000 historic shipwrecks estimated to be in the waters between Greenland and the Svalbard archipelago (Guijarro Garcia et al., 2006), the area could potentially be a treasure trove of information not only on Svalbard history but also on 400 years of Europe’s richest maritime history. The presence of wood-boring bivalves may pose a hitherto unrecognized threat to this underwater record of centuries of extractive activity along the Arctic frontier. The newly discovered wreck of Figaro, a wooden whaling ship that sank in 1908, did not show significant signs of damage from wood-boring organisms (Mogstad et al., 2020). Figaro was discovered in Isfjorden on the west coast of Svalbard ~100 nm south of Smeerenburg (Figure 1; for details regarding Figaro, see Mogstad et al., 2020). The fact that Figaro presently is the only investigated historical wreck in the Svalbard archipelago underscores the profound knowledge gaps related to the natural and cultural history of the seabed in these areas.
The rate of Arctic warming is to two to four times the global average (Gerland et al., 2023), which will impact the biological diversity as we know it today. A likely effect will be to extend the distribution of boreal species northward. Following this, the entry of new and more wood-boring organisms to the Arctic will pose a threat to cultural heritage, as observed in this story. However, the story and future perspectives may be entirely different for the deep-sea, cold-water species Xyloredo nooi. The combination of less sea ice and a much-reduced timber industry in Siberia is likely to result in reduced substrate for these species. To some extent, this may be partly counteracted by faster flow of the TPD (Figure 1), reducing the potential time it takes for a piece of wood to be transported across the Arctic Ocean. Nevertheless, less wood on the seabed will then reduce the availability of steppingstones for wood-boring organisms. On the other hand, more frequent extreme weather events as a result of global climate change could increase wood input to the ocean. All of these factors combined, and in the light of the present knowledge, it is difficult to predict the status and the vulnerability of these deep-sea organisms.
Summary and Conclusions
The report of wood-boring mollusks in the High Arctic is indicative of a hitherto unknown, but potentially ecologically significant, element of the Arctic marine biota. Collected in the relatively well-studied fjords of Svalbard, the discovery also points toward a major gap in knowledge regarding biodiversity and ecosystem composition. Such knowledge gaps are particularly relevant in light of the Norwegian government’s recent decision to allow exploration and mapping of the seafloor in preparation for future development of deep-sea mining (Nature, 2024).
Climate change is fundamentally transforming the Arctic. Half of the summer sea ice has disappeared since the 1980s, and the rest is projected to be gone within the coming decades (Kim et al., 2023). The warming extends from the deep ocean to the upper atmosphere, impacting ocean circulation, weather patterns, ecosystems, and human presence in the region (Gerland et al., 2023; Nanni et al., 2024). We need to close knowledge gaps concerning the Arctic biota to understand the present composition of Arctic benthic organisms and their ecosystems and to understand and manage changes to these areas. As demonstrated in this study, the natural and cultural histories of the Arctic are deeply intertwined, necessitating interdisciplinary approaches to uncover connections and insights across domains that might otherwise remain obscure. Climate change coinciding with increased interest from commercial and geopolitical actors in the region further enhance this need.
Acknowledgments
The authors are grateful to UiT The Arctic University of Norway for funding the two research cruises, and to the crew onboard FF Helmer Hanssen for all their help and assistance. The authors also want to thank the editor and two anonymous reviewers for providing constructive comments in two rounds of review.
Citation
Berge, J., T. Bakken, K. Heggland, J.-A. Sneli, Ø. Ødegård, M. Ingulstad, T. Thun, and G. Johnsen. 2025. Exploring climate change, geopolitics, marine archeology, and ecology in the Arctic Ocean through wood-boring bivalves. Oceanography 38(2):52–57, https://doi.org/10.5670/oceanog.2025.311.
References
- Alm, T. 2019. Drivved og drivtømmer i norsk folketradisjon (Norwegian), Drift wood and timber in Norwegian folk tradition. Blyttia 77:247–270.
- Aniol, R.W. 1983. Tree-ring analysis using CATRAS. Dendrochronologia 1:45–53.
- Baillie, M.G.L. 1982. Tree-Ring Dating and Archaeology. Croom Helm, London, 274 pp.
- Baillie, M.G.L., and J.R. Pilcher. 1973. A simple crossdating program for tree-ring research. Tree-Ring Bulletin 33:7–14.
- Bakken, T., K. Hårsaker, and M. Daverdin. 2024. Marine invertebrate collection NTNU University Museum. Version 1.1899. Norwegian University of Science and Technology. Occurrence dataset accessed via GBIF.org on 2024-11-16.
- Berge, J., G. Johnsen, F. Nilsen, B. Gulliksen, and D. Slagstad. 2005. Ocean temperature oscillations enable reappearance of blue mussels Mytilus edulis in Svalbard after a 1000 year absence. Marine Ecology Progress Series 303:167–175, https://doi.org/10.3354/meps303167.
- Berge, J., F. Cottier, K.S. Last, Ø. Varpe, E. Leu, J. Soreide, K. Eiane, S. Falk-Petersen, K. Willis, H. Nygard, D. Vogedes, C. Griffiths, G. Johnsen, D. Lorentzen, and A.S. Brierley. 2009. Diel vertical migration of Arctic zooplankton during the polar night. Biology Letters 5:69–72, https://doi.org/10.1098/rsbl.2008.0484.
- Berge, J., P. Anderson, P. Kupiszewski, M. Granskog, M. Bratrein, D. Divine, P. De La Torre, M. Geoffroy, A. Zolich, and T. Kopec. 2025. “Data from Drifting, Autonomous Ice-Tethered Observatories Deployed in June 2022 at 89.82N, 26.28W.” Norwegian Meteorological Institute, https://doi.org/10.21343/WJ48-Y250.
- Cottier, F., R. Skogseth, D. David, F. de Rovere, D. Vogedes, M. Daase, and J. Berge. 2021. Temperature and salinity time series in Svalbard fjords – ‘Integrated marine observatory partnership (iMOP II).’ Pp. 26–36 in SESS report 2021 - The state of environmental science in Svalbard - An annual report Svalbard Integrated Arctic Earth Observing System (2022).
- Cottier, F., J. Berge, C. Griffith, E. Dumont, T.P. Kopec, and D. Vogedes. 2022.Temperature, salinity, light and fluorescence (CTD) measurements from the Kongsfjorden (Svalbard) marine observatory (mooring) September 2008–August 2009 [Data set]. Norstore, https://doi.org/10.11582/2022.00026.
- Distel, D.L., M. Amin, A. Burgoyne, E. Linton, G. Mamangkey, W. Morrill, J. Nove, N. Wood, and J. Yang. 2011. Molecular phylogeny of Pholadoidea Lamarck, 1809 supports a single origin for xylotrophy (wood feeding) and xylotrophic bacterial endosymbiosis in Bivalvia. Molecular Phylogenetics and Evolution 61(2):245–254, https://doi.org/https://doi.org/10.1016/j.ympev.2011.05.019.
- Eckstein, D. 2007. Human time in tree rings. Dendrochronologia 24(2):53–60, https://doi.org/10.1016/j.dendro.2006.10.001.
- Eggertsson, O. 1994. Driftwood as an indicator of relative changes in the influx of Arctic and Atlantic water into the coastal areas of Svalbard. Polar Research 13(2):209–218, https://doi.org/10.3402/polar.v13i2.6694.
- Gerland, S., R.B. Ingvaldsen, M. Reigstad, A. Sundfjord, B. Bogstad, M. Chierici, H. Hop, P.E. Renaud, L.H. Smedsrud, L.C. Stige, and others. 2023. Still Arctic? The changing Barents Sea. Elementa Science of the Anthropocene 11(1):00088, https://doi.org/10.1525/elementa.2022.00088.
- Goodell, B., J. Chambers, D.V. Ward, C. Murphy, E. Black, L.B.K. Mancilio, G. Perez-Gonzalez, and J.R. Shipway. 2024. First report of microbial symbionts in the digestive system of shipworms; wood boring mollusks. International Biodeterioration & Biodegradation 192:105816, https://doi.org/10.1016/j.ibiod.2024.105816.
- Guijarro Garcia, E., S. Ragnarsson, S. Steingrimsson, D. Nævestad, H. Haraldsson, J.H. Fosså, O.S. Tendal, and H. Eiriksson. 2006. Bottom trawling and scallop dredging in the Arctic: Impacts of fishing on non-target species, vulnerable habitats, and cultural heritage. Nordic Council of Ministers, Copenhagen.
- Häggblom, A. 1982. Driftwood in Svalbard as an indicator of sea ice conditions: A preliminary report. Geografiska Annaler: Series A, Physical Geography 64(1–2):81–94, https://doi.org/10.1080/04353676.1982.11880057.
- Hellmann, L., W. Tegel, A.V. Kirdyanov, O. Eggertsson, J. Esper, L. Agafonov, A.N. Nikolaev, A.A. Knorre, V.S. Myglan, O. Churakova, and others. 2015. Timber logging in Central Siberia is the main source for recent Arctic driftwood. Arctic, Antarctic and Alpine Research 47(3):449–460, https://doi.org/10.1657/AAAR0014-063.
- Ingvaldsen, R.B., E. Eriksen, T. Haug, and H.R. Skjoldal. 2024. State, variability, and trophic interactions in the Atlantic gateway to the Arctic. Progress in Oceanography 226:103276, https://doi.org/10.1016/j.pocean.2024.103276.
- Jordà-Molina, E., P.E. Renaud, M.J. Silberberger, A. Sen, B.A. Bluhm, M.L. Carroll, W.G. Ambrose Jr., F. Cottier, and H. Reiss. 2023. Seafloor warm water temperature anomalies impact benthic macrofauna communities of a high-Arctic cold-water fjord. Marine Environmental Research 189:106046, https://doi.org/10.1016/j.marenvres.2023.106046.
- Kim, Y.H., S.K. Min, N.P. Gillett, D. Notz, and E. Malinina. 2023. Observationally-constrained projections of an ice-free Arctic even under a low emission scenario. Nature Communications 14:3139, https://doi.org/10.1038/s41467-023-38511-8.
- Kolar, T., M. Rybnicek, P.E. Aspholm, P. Cermak, O. Eggertsson, V. Gryc, T. Zid, and P. Buntgen. 2022. The importance of Arctic driftwood for interdisciplinary global change research. Czech Polar Reports 12(2):172–180, https://doi.org/10.5817/CPR2022-2-13.
- Korpachev, V.P., A.I. Perezhilin, A.A. Andriyas, and M.D. Ivanov. 2022. Technologies of cleaning from wood of the mouth of the Yenisei River. Conifers of the Boreal Area XL(6):487–494.
- Kotchetkova, E. 2004. The Green Power of Socialism: Wood, Forest, and the Making of Soviet Industrially Embedded Ecology. MIT Press, Cambridge, MA.
- Kuksin, I.Y. 1991. The Arctic Ocean hydrographic expedition 1910–1915. Polar Geography and Geology 15(4):299–309, https://doi.org/10.1080/10889379109377467.
- Linderholm, H.W., B.E. Gunnarson, M. Fuentes, U. Büntgen, and A. Hormes. 2021. The origin of driftwood on eastern and south-western Svalbard. Polar Science 29:100658, https://doi.org/10.1016/j.polar.2021.100658.
- Lotz, C. 2015. Expanding the space for future resource management: Explorations of the timber frontier in Northern Europe and the rescaling of sustainability during the nineteenth century. Environment and History 21(2):257–279, https://doi.org/10.3197/096734015X14267043141462.
- McClain, C.R., D. Amon, M. Bowles, S.R.D Bryant, G. Hanks, S. McDermott, E. Thomas, and E. Young. 2025. The hidden forests below: A review of the ecology and evolution of wood falls on the deep seafloor. Marine Ecology 46:e70008, https://doi.org/10.1111/maec.70008.
- Mogstad, A., Ø. Ødegård, S.M. Nornes, M. Ludvigsen, G. Johnsen, A.J. Sørensen, and J. Berge. 2020. Mapping the historical shipwreck Figaro in the High Arctic using underwater sensor-carrying robots. Remote Sensing 12:997, https://doi.org/10.3390/rs12060997.
- Nansen, F. 1897. Farthest North. Being the Record of a Voyage of Exploration of the Ship “Fram” 1893–96 and of a Fifteen Months’ Sleigh Journey by Dr. Nansen and Lieut. Johansen, Vol. I. Harper & Brothers, New York.
- Nahrgang, J., Ø. Varpe, E. Korshunova, S. Murzina, I.G. Hallanger, I. Vieweg, and J. Berge. 2014. Gender specific reproductive strategies of an Arctic key species (Boreogadus saida) and implications of climate change. PLoS ONE 9(5):e98452, https://doi.org/10.1371/journal.pone.0098452.
- Nanni, U., P. DeRepentigny, A. Lundén, V. Popovaitė, Y. Shen, I.K. Basaran, N.D. Neubern, L. Mascorda-Cabre, A. Bennett, T. Vold Hansen, and others. 2024. Redefining Arctic boundaries in a changing climate: Interdisciplinary perspectives on governance strategies. Polar Geography 47(2):127–155, https://doi.org/10.1080/1088937X.2024.2359926.
- Nature. 2024. Norway’s approval of sea-bed mining undermines efforts to protect the ocean. Nature (editorial) 625:424, https://doi.org/10.1038/d41586-024-00104-w.
- Naumov, V., P. Angelstam, and M. Elbakidze. 2016. Barriers and bridges for intensified wood production in Russia: Insights from the environmental history of a regional logging frontier. Forest Policy and Economics 66:1–10, https://doi.org/10.1016/j.forpol.2016.02.001.
- Nielsen, J.P., and E. Okhuizen, eds. 2022. From Northeast Passage to Northern Sea Route: A History of the Waterway North of Eurasia. Brill, Leiden.
- O’Connor, R.M., J.M. Fung, K.H Sharp, J.S. Benner, C. McClung, S. Cushing, E.R. Lamkin, A.I. Fomenkov, B. Henrissat, Y.Y. Londer, and others. 2014. Gill bacteria enable a novel digestive strategy in a wood-feeding mollusk. Proceedings of the National Academy of Sciences of the United States of America 111:E5096–E5104, https://doi.org/10.1073/pnas.1413110111.
- Purchon, R.D. 1941. On the biology and relationships of the lamellibranch Xylophaga Dorsalis (Turton). Journal of the Marine Biological Association of the United Kingdom 25:1–39.
- Reymert, P.K., and O. Moen. 2015. Fangsthytter på Svalbard 1794–2015 Svalbard museum. Longyearbyen.
- Ristova, P., C. Bienhold, F. Wenzhöfer, P.E. Rossel, and A. Boetius. 2017. Temporal and spatial variations of bacterial and faunal communities associated with deep-sea wood falls. PLoS ONE 12(1):e0169906, https://doi.org/10.1371/journal.pone.0169906.
- Romano, C., A. Nunes-Jorge, N. Le Bris, G.W. Rouse, D. Martin, and C. Borowski. 2020. Wooden stepping stones: Diversity and biogeography of deep-sea wood boring Xylophagaidae (Mollusca: Bivalvia) in the North-East Atlantic Ocean, with the description of a new genus. Frontiers in Marine Science 7:579959, https://doi.org/10.3389/fmars.2020.579959.
- Spall, M.A. 2019. Dynamics and thermodynamics of the mean Transpolar Drift and ice thickness in the Arctic Ocean. Journal of Climate 32:8,449–8,463, https://doi.org/10.1175/JCLI-D-19-0252.1.
- Stewart, J., L.D. Murdock, and P. Waddell. 1995. Reburial of the Red Bay wreck as a form of preservation and protection of the historic resource. MRS Online Proceedings Library 352:791.
- Thun, T., and J.M. Stornes. 2016. Dendrochronology brings new life to the stave churches. Dating and material analysis. Pp. 96–116 in Preserving the Stave Churche: Craftmanship and Research. K. Bakken, ed., Pax forlag, Oslo.
- Turner, R.D. 1972. Xyloredo, a new terenidid-like abyssal wood-borer (Mollusca, Pholadidae, Xylophaginae). Breviora 397:1–19.
- Turner, R.D. 2002. On the subfamily Xylophagainae (Family Pholadidae, Bivalvia, Mollusca). Bulletin of the Museum of Comparative Zoology 157:223–307.
- Voight, J.R. 2015. Xylotrophic bivalves: Aspects of their biology and the impacts of humans. Journal of Molluscan Studies 81(2):175–186, https://doi.org/10.1093/mollus/eyv008.
- Voight, J.R. 2022. Species synonymies in the deep-sea wood-boring bivalve genus Xyloredo (Mollusca: Xylophagaidae). Malacologia 64(2):163–168, https://doi.org/10.4002/040.064.0201.
Copyright & Usage
This is an open access article made available under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution, and reproduction in any medium or format as long as users cite the materials appropriately, provide a link to the Creative Commons license, and indicate the changes that were made to the original content. Images, animations, videos, or other third-party material used in articles are included in the Creative Commons license unless indicated otherwise in a credit line to the material. If the material is not included in the article’s Creative Commons license, users will need to obtain permission directly from the license holder to reproduce the material.